pd催化不对称合成手性2-三氟甲基-4-(吲哚-3-基)- 4h -铬衍生物
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
本文提出了一种构建手性4h -铬骨架的新策略。通过钯催化的2-三氟甲基-4-(吲哚-3-基)- 4h -铬的不对称缩合反应,以中高收率(60-92%)合成了一系列手性2-三氟甲基-4-(吲哚-3-基)- 4h -铬,其对映选择性高达97% ee。这些三氟甲基化、立体化学丰富的构建块在药物化学中具有潜在的价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
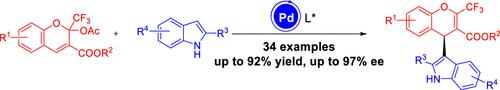
Pd-Catalyzed Asymmetric Synthesis of Chiral 2-Trifluoromethyl-4-(indol-3-yl)-4H-chromene Derivatives
This paper presents a new strategy for the construction of the chiral 4H-chromene skeleton. A series of chiral 2-trifluoromethyl-4-(indol-3-yl)-4H-chromenes were synthesized in moderate to good yields (60–92%) with excellent enantioselectivity (up to 97% ee) through the palladium-catalyzed asymmetric condensation of 2H-chromenes and indoles. These trifluoromethylated, stereochemically rich building blocks hold potential value in medicinal chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


