细菌及其衍生物在肿瘤靶向治疗中的创新应用
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
尽管癌症治疗取得了重大进展,但传统疗法仍然面临着相当大的挑战,包括靶向性差、严重的毒副作用和耐药性的发展。生物技术的最新进展揭示了细菌及其衍生物利用其生物学特性作为肿瘤治疗药物传递系统的潜力。包括大肠杆菌、沙门氏菌和单核增生李斯特菌在内的工程细菌及其衍生物──外膜囊泡(OMVs)、细菌幽灵(BGs)和细菌孢子(BSPs)──可以装载多种抗肿瘤药物,从而实现肿瘤微环境(TME)内的精确靶向和持续药物释放。这些细菌及其衍生物具有刺激免疫系统的内在特性,增强先天和适应性免疫反应,进一步扩大治疗效果。细菌在缺氧肿瘤区域自然积累的能力及其基因修饰的多功能性允许定制药物递送策略,协同提高化疗,免疫治疗和靶向治疗的有效性。本文综述了细菌治疗的基本原理,重点介绍了细菌工程、药物装载、细菌及其衍生物在靶向肿瘤治疗中的应用。它还讨论了优化细菌传递系统所面临的挑战,如安全性问题、意外免疫反应和临床应用的可扩展性。通过对这些方面的探索,本综述为改进基于细菌的药物传递系统提供了理论框架,有助于开发更有效和个性化的癌症治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
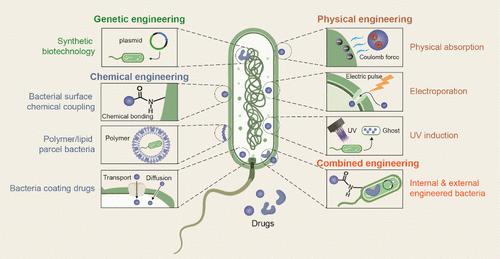
Innovative Applications of Bacteria and Their Derivatives in Targeted Tumor Therapy
Despite significant progress in cancer treatment, traditional therapies still face considerable challenges, including poor targeting, severe toxic side effects, and the development of resistance. Recent advances in biotechnology have revealed the potential of bacteria and their derivatives as drug delivery systems for tumor therapy by leveraging their biological properties. Engineered bacteria, including Escherichia coli, Salmonella, and Listeria monocytogenes, along with their derivatives─outer membrane vesicles (OMVs), bacterial ghosts (BGs), and bacterial spores (BSPs)─can be loaded with a variety of antitumor agents, enabling precise targeting and sustained drug release within the tumor microenvironment (TME). These bacteria and their derivatives possess intrinsic properties that stimulate the immune system, enhancing both innate and adaptive immune responses to further amplify therapeutic effects. The ability of bacteria to naturally accumulate in hypoxic tumor regions and their versatility in genetic modifications allow for tailored drug delivery strategies that synergistically enhance the effectiveness of chemotherapy, immunotherapy, and targeted therapies. This review comprehensively examines the fundamental principles of bacterial therapy, focusing on the strategies employed for bacterial engineering, drug loading, and the use of bacteria and their derivatives in targeted tumor therapy. It also discusses the challenges faced in optimizing bacterial delivery systems, such as safety concerns, unintended immune responses, and scalability for clinical applications. By exploring these aspects, this review provides a theoretical framework for improving bacterial-based drug delivery systems, contributing to the development of more effective and personalized cancer treatments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


