肿瘤缺氧驱动基因组不稳定性和肿瘤进化
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
瘤内缺氧是所有非均质实体瘤的一个特征。肿瘤缺氧水平或亚区增加与不良临床预后相关,特别是当这种情况与基因组不稳定同时发生时。实验证据表明,增殖缺氧细胞的DNA获取和染色体改变继发于DNA修复途径的抑制,如同源重组、碱基切除修复和错配修复。修复缺陷细胞中的细胞适应和选择产生了一种模式,即新的单核苷酸突变、结构变异和拷贝数改变与有丝分裂控制改变共存,从而驱动染色体不稳定和非整倍体。全基因组测序研究支持这样一种观点,即缺氧与MYC、BCL2、TP53和PTEN的驱动突变一起,是决定多种肿瘤类型克隆和亚克隆进化的关键微环境辅助因素。我们认为,低氧肿瘤微环境选择了不稳定的肿瘤克隆,这些克隆在免疫监视降低的情况下存活、繁殖和转移。缺氧肿瘤细胞的这些侵袭性特征支持对局部和全身治疗的抵抗,并对癌症患者产生不利的结果。可能的方法来对抗缺氧的影响,以阻止肿瘤进化和改善治疗结果描述。本文章由计算机程序翻译,如有差异,请以英文原文为准。

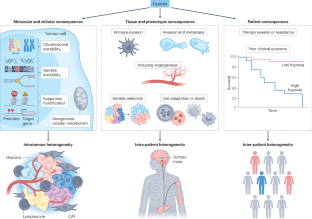
Tumour hypoxia in driving genomic instability and tumour evolution
Intratumour hypoxia is a feature of all heterogenous solid tumours. Increased levels or subregions of tumour hypoxia are associated with an adverse clinical prognosis, particularly when this co-occurs with genomic instability. Experimental evidence points to the acquisition of DNA and chromosomal alterations in proliferating hypoxic cells secondary to inhibition of DNA repair pathways such as homologous recombination, base excision repair and mismatch repair. Cell adaptation and selection in repair-deficient cells give rise to a model whereby novel single-nucleotide mutations, structural variants and copy number alterations coexist with altered mitotic control to drive chromosomal instability and aneuploidy. Whole-genome sequencing studies support the concept that hypoxia is a critical microenvironmental cofactor alongside the driver mutations in MYC, BCL2, TP53 and PTEN in determining clonal and subclonal evolution in multiple tumour types. We propose that the hypoxic tumour microenvironment selects for unstable tumour clones which survive, propagate and metastasize under reduced immune surveillance. These aggressive features of hypoxic tumour cells underpin resistance to local and systemic therapies and unfavourable outcomes for patients with cancer. Possible ways to counter the effects of hypoxia to block tumour evolution and improve treatment outcomes are described. In this Review, Suvac et al. discuss how intratumoural hypoxia is a driving force in tumour evolution, alongside driver gene mutations, through the generation of genomic instability. The resultant selected unstable tumour clones underpin resistance to local and systemic therapies and unfavourable outcomes for patients with cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


