癌症免疫学和免疫治疗中的交叉启动
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
细胞毒性T细胞对癌症的免疫应答主要依赖于一种称为常规1型树突状细胞(cDC1s)的专业抗原呈递细胞亚型交叉呈递抗原的能力。交叉呈递包括将来自其他细胞的外源抗原重定向到主要的组织相容性复合体I类抗原呈递机制。此外,一旦被激活,并通过CD40-CD40L相互作用感知病毒部分或T辅助细胞合作,cDC1s提供关键的共刺激配体和细胞因子,以建立和维持CD8+ T细胞免疫应答。这种同源T细胞活化的调控过程被称为交叉启动。在癌症小鼠模型中,cDC1s交叉启动CD8+ T细胞对大多数(如果不是全部的话)免疫治疗策略的疗效至关重要。在癌症患者中,肿瘤微环境中cDC1s的存在和丰度与T细胞浸润水平和对免疫检查点抑制剂的反应性显著相关。利用fms样酪氨酸激酶3配体(FLT3L)和/或它们的激活状态来增加cDC1s数量的治疗策略在癌症小鼠模型中显示出有效性的证据,目前正在进行初步临床试验,迄今为止结果令人鼓舞。本文章由计算机程序翻译,如有差异,请以英文原文为准。

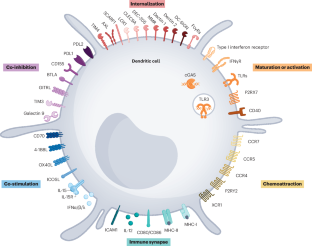
Cross-priming in cancer immunology and immunotherapy
Cytotoxic T cell immune responses against cancer crucially depend on the ability of a subtype of professional antigen-presenting cells termed conventional type 1 dendritic cells (cDC1s) to cross-present antigens. Cross-presentation comprises redirection of exogenous antigens taken from other cells to the major histocompatibility complex class I antigen-presenting machinery. In addition, once activated and having sensed viral moieties or T helper cell cooperation via CD40–CD40L interactions, cDC1s provide key co-stimulatory ligands and cytokines to mount and sustain CD8+ T cell immune responses. This regulated process of cognate T cell activation is termed cross-priming. In cancer mouse models, CD8+ T cell cross-priming by cDC1s is crucial for the efficacy of most, if not all, immunotherapy strategies. In patients with cancer, the presence and abundance of cDC1s in the tumour microenvironment is markedly associated with the level of T cell infiltration and responsiveness to immune checkpoint inhibitors. Therapeutic strategies to increase the numbers of cDC1s using FMS-like tyrosine kinase 3 ligand (FLT3L) and/or their activation status show evidence of efficacy in cancer mouse models and are currently being tested in initial clinical trials with promising results so far. In this Review, Luri-Rey et al. present evidence for the crucial role of conventional type 1 dendritic cells in cross-presenting antigens from other cells via the major histocompatibility complex class I antigen-presenting machinery, which in turn is necessary to prime CD8+ T cells, which then mount efficient immune responses against cancer. The authors also discuss how we can exploit these processes in cancer immunotherapy by increasing the number and/or maturation or activation status of this specialist subtype of antigen-presenting cell.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


