对产生含有非天然氨基酸的多种二肽的 ATP-Grasp肽连接酶底物识别机制的结构性见解
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
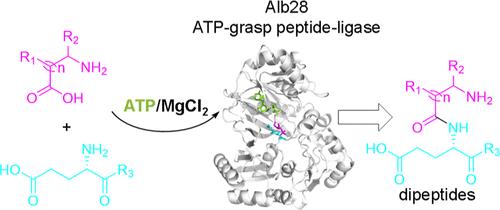
Structural Insights into the Substrate Recognition Mechanism of an ATP-Grasp Peptide-Ligase Producing Diverse Dipeptides Containing Unnatural Amino Acids
ATP-grasp enzymes are involved in many different biological processes of primary and secondary metabolism and catalyze the production of many physiological or medicinally valuable peptides. Here, we report an ATP-grasp enzyme Alb28, from Streptomyces albogriseolus MGR072, which exhibits broad substrate promiscuity and strict stereoselectivity, thereby capable of producing at least 55 structurally diversified dipeptides, greatly expanding the existing dipeptide library. By combining crystallographic studies, molecular dynamics simulations, and mutagenesis assays, we identified the critical residues in Alb28 responsible for regulating the substrate recognition and enzymatic catalysis. Particularly, two structural motifs in Alb28 are involved in dictating the entry of substrate into the active-site via the opening/closing motions. Our work potentiates the future applications of Alb28 in generating structurally diversified dipeptides for therapeutic usages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


