加速剪接转换寡核苷酸的内体逸出可实现高效肝脏剪接校正
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
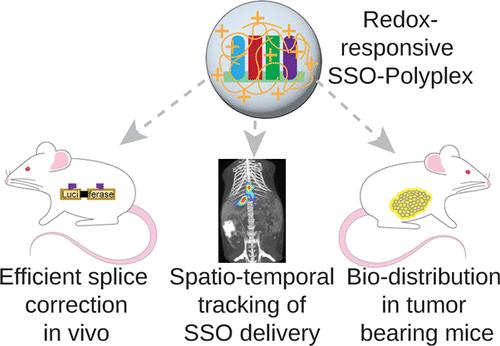
Accelerated Endosomal Escape of Splice-Switching Oligonucleotides Enables Efficient Hepatic Splice Correction
Splice-switching oligonucleotides (SSOs) can restore protein functionality in pathologies and are promising tools for manipulating the RNA-splicing machinery. Delivery vectors can considerably improve SSO functionality in vivo and allow dose reduction, thereby addressing the challenges of RNA-targeted therapeutics. Here, we report a biocompatible SSO nanocarrier, based on redox-responsive disulfide cross-linked low-molecular-weight linear polyethylenimine (cLPEI), for overcoming multiple biological barriers from subcellular compartments to en-route serum stability and finally in vivo delivery challenges. Intracellularly responsive cross-links of cLPEI significantly accelerated the endosomal escape and offered efficient SSO release to the cell’s nucleus, thereby leading to high splice correction in vitro. In vivo performance of cLPEI-SSOs was investigated in a novel transgenic mouse model for splice correction, spatiotemporal tracking of SSO delivery in wild-type mice, and biodistribution in a colorectal cancer peritoneal metastasis model. A single intravenous application of 5 mg kg–1 cLPEI-SSOs induced splice correction in liver, lung, kidney, and bladder, giving functional protein, which was validated by RT-PCR. Near-infrared (NIR) fluorescence imaging and X-ray computed tomography revealed improved organ retention and reduced renal excretion of SSOs. NIR microscopy demonstrated the accumulation of SSOs in angiogenic tumors within the pancreas. Successful nuclear delivery of SSOs was observed in the hepatocytes. Thus, cLPEI nanocarriers resulted in highly efficient splice correction in vivo, highlighting the critical role of the enhanced SSO bioavailability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


