锰介导叔胺自由基级联环化合成多取代1,2-二氢吡啶和吡啶
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
研究了Mn(III)介导的n -丙炔酰胺与亚硫酸钠的自由基级联环化反应。机理研究表明,该级联过程主要由磺基自由基在C≡C键上的化学选择性加成和分子内6-内三环的区域选择性组成。该方案仅通过改变溶剂和温度,就为多种多取代间磺酰基吡啶或1,2-二氢吡啶的发散合成提供了强有力的方法,具有良好的官能团相容性和简单的操作。本文章由计算机程序翻译,如有差异,请以英文原文为准。
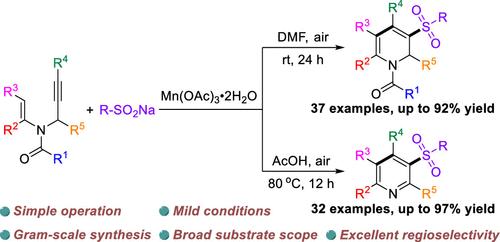
Divergent Synthesis of Polysubstituted 1,2-Dihydropyridines and Pyridines through Manganese-Mediated Radical Cascade Cyclization of Tertiary Enamides
A Mn(III)-mediated radical cascade cyclization of N-propargyl enamides with sodium sulfinates was developed. Mechanistic studies indicated that this cascade process is mainly composed of the chemoselective addition of sulfonyl radical to C≡C bond and the regioselective intramolecular 6-endo-trig cyclization. This protocol provided a powerful method for the divergent synthesis of diverse polysubstituted meta-sulfonylpyridines or 1,2-dihydropyridines just by varying solvent and temperature featuring good functional group compatibility and simple operation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


