炎症微环境中的自噬激活提高了msc来源的细胞外囊泡对SLE的治疗效果
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
开发策略以提高间充质干细胞(MSC)衍生的细胞外囊泡(EVs)在自身免疫性疾病中的治疗效果已引起越来越多的关注。目的探讨雷帕霉素诱导的系统性红斑狼疮(SLE)炎症微环境(Rapa-SLE)自噬是否能增强间充质干细胞来源的ev对SLE的治疗作用。方法在MRL/lpr小鼠中,对制备的rapa - slee - ev的治疗潜力进行评价。将rapa -SLE- ev与用去ev胎牛血清(FBS- ev)培养的MSCs衍生的ev、用雷帕霉素处理的FBS培养的MSCs (Rapa-FBS-EV)培养的ev以及暴露于不含雷帕霉素的SLE血清(SLE- ev)的ev进行比较。评估各组小鼠的自身免疫反应、肾功能和病理损伤。此外,对抗炎蛋白IDO1在EVs中的作用进行了机制研究。结果与SLE炎症微环境的相互作用触发了间充质干细胞的自噬,雷帕霉素治疗进一步增强了自噬。Rapa-SLE-EV可显著改善MRL/lpr小鼠的自身免疫反应和肾脏损害,优于其他MSC-EV组。这种治疗减轻了SLE的主要表现,包括自身抗体水平降低、脾肿大和淋巴结病。此外,Rapa-SLE-EV表现出较好的血浆炎症因子抑制作用,保留肾功能,减轻病理性损伤,减少肾小球免疫复合物沉积。在机制上,Rapa-SLE-EV对SLE-B细胞功能表现出特殊的抑制作用,这得益于抗炎蛋白IDO1的高表达,该蛋白已被证实通过ev进入SLE-B细胞。结论:我们提出了一种新的策略来提高msc - ev在SLE中的治疗效果,并证实msc - ev的免疫调节功能通过自噬激活和与SLE血清微环境的相互作用而增强,这一过程可能得益于IDO1的高表达。本文章由计算机程序翻译,如有差异,请以英文原文为准。
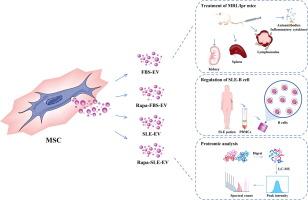
Autophagy activation within inflammatory microenvironment improved the therapeutic effect of MSC-Derived extracellular Vesicle in SLE
Introduction
Developing strategies to improve the therapeutic efficacy of mesenchymal stem cell (MSC)-derived extracellular vesicles (EVs) in autoimmune diseases have garnered increased attention.Objectives
To evaluate whether rapamycin-induced autophagy within the systemic lupus erythematosus (SLE) inflammatory microenvironment (Rapa-SLE) augments the therapeutic effects of MSC-derived EVs in SLE.Methods
The therapeutic potential of the resulting EVs (Rapa-SLE-EV) was assessed in MRL/lpr mice. Rapa-SLE-EVs were compared with EVs derived from MSCs from MSCs cultured with EV-depleted fetal bovine serum (FBS-EV), EVs from MSCs cultured with rapamycin-treated FBS (Rapa-FBS-EV), and EVs exposed to SLE serum without rapamycin (SLE-EV). The autoimmune response, renal function, and pathological damage were assessed among the mouse groups. Additionally, mechanistic investigations into the role of the anti-inflammatory protein IDO1 within the EVs.Results
Interaction with the SLE inflammatory microenvironment triggered autophagy in MSCs, which was further enhanced by rapamycin treatment. Rapa-SLE-EV administration significantly ameliorated the autoimmune response and renal damage in MRL/lpr mice, outperforming other MSC-EV groups. This treatment mitigated key manifestations of SLE, including reduced autoantibody levels, as well as splenomegaly, and lymphadenopathy. Furthermore, Rapa-SLE-EV demonstrated superior suppression of plasma inflammatory cytokines, preserved renal function, mitigated pathological damage, and reduced glomerular immune complex deposition. Mechanistically, Rapa-SLE-EV exhibits exceptional inhibitory effects on SLE-B cell function, benefited by the high expression of the anti-inflammatory protein IDO1, which was confirmed to enter SLE-B cells through EVs.Conclusions
We developed a novel strategy to improve the therapeutic efficacy of MSC-EVs in SLE and confirmed that the immunomodulatory function of the MSC-EVs is enhanced through autophagic activation and interaction with the SLE serum microenvironment, a process likely benefited by the high expression of IDO1.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


