密实生长和缓冲作用,使锌阳极稳定可逆
IF 17.1
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
电极/电解质界面的化学环境对锌离子沉积行为和与水有关的寄生副反应至关重要。本研究提出以咪唑基团组氨酸作为多功能电解质添加剂来解决枝晶形成、析氢和腐蚀等问题。组氨酸在Zn(100)和Zn(101)平面上表现出较强的优先吸附作用,导致Zn(002)面暴露,从而促进Zn(002)平面的密实生长,实现无枝晶沉积。咪唑基团既能接受质子又能释放质子,调节界面质子浓度,从而有效抑制析氢反应和不良副产物的形成。添加0.1 M组氨酸的Zn||锌对称细胞表现出良好的循环稳定性,在1 mA cm⁻²下的运行时间超过3200 h,远远超过不添加锌的细胞(50 h后短路)。在1400次循环中,Zn||Cu不对称电池的库仑效率高达99.8 %。经过200次循环后,Zn|| nh4v4010袋状电池可稳定运行,容量保持率为80% %。本文章由计算机程序翻译,如有差异,请以英文原文为准。
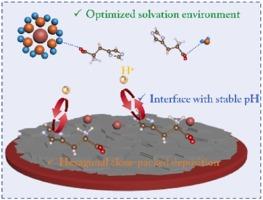
Close-packed growth and buffer action enabling stable and reversible Zn anode
The chemical environment at the electrode/electrolyte interface is critical for the zinc ions deposition behavior and water-related parasitic side reactions. In this study, histidine, with imidazole group, is proposed as a multifunctional electrolyte additive to address the issues like dendrite formation, hydrogen evolution, and corrosion. The histidine exhibits a strong preferential adsorption on the Zn(100) and Zn(101) planes, leading to the exposure of Zn(002) facet, thereby promoting the close-packed growth along (002) plane and achieving a dendrite-free deposition. The imidazole group can both accept and release protons, which regulates the interfacial proton concentration, thus effectively suppressing hydrogen evolution reaction and the formation of undesirable by-products. The Zn||Zn symmetric cell with 0.1 M histidine exhibits good cycling stability, with over 3200 h of operation at 1 mA cm⁻², far surpassing cells without the additive (short circuit after 50 h). The Zn||Cu asymmetric cells demonstrate a high Coulombic efficiency of 99.8 % over 1400 cycles. The Zn||NH4V4O10 pouch cell can be steadily operated with 80 % capacity retention after 200 cycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


