用于选择性吸附全氟辛烷磺酸的氟化 LDH:揭示疏水性增强和 F-F 相互作用的作用
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。

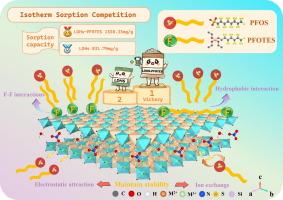
Fluorinated LDHs for selective sorption of PFOS: Unveiling the roles of increased hydrophobicity and F-F interactions
Layered double hydroxides have been proved to be effective adsorbents for the removal of per- and polyfluoroalkyl substances (PFASs) from aqueous solutions. In this study, a novel layered double hydroxides modified by perfluorooctyltriethoxysilane (LDHs-PFOTES) was synthesized at room temperature, demonstrating rapid and selective sorption of perfluorooctane sulfonic acid (PFOS). The covalent grafting of fluorosilane groups onto the LDHs surface enhanced hydrophobicity and introduced fluorine functional groups, while preserving the positive charge essential for electrostatic interactions. LDHs-PFOTES removed 97.21 % of PFOS from water within 5 min, with a sorption capacity of 1530.33 mg/g, nearly doubling the capacity of unmodified LDHs (831.79 mg/g). The thermodynamic analysis of PFOS sorption on LDHs-PFOTES revealed that the process is spontaneous and endothermic. LDHs-PFOTES maintained over 90 % PFOS removal efficiency under varying pH levels, ionic strengths, and the presence of competing compounds. Furthermore, the C-F structure on the LDHs-PFOTES enabled fluorine-fluorine (F-F) interactions with PFOS, further improving selective sorption. It was concluded that the combined effects of electrostatic interactions, hydrophobicity, F-F interactions, and ion exchange resulted in the efficient and selective removal of PFOS from water. LDHs-PFOTES demonstrated 95.37 % sorptive efficiency in removing PFOS when treating a mixture of 11 PFAS compounds. Additionally, LDHs-PFOTES effectively desorbed PFOS using a mixed solution of NaCl and methanol, maintaining approximately 95.08 % removal efficiency over three regeneration cycles. This fluorination strategy to modify LDHs offers a novel approach for selectively and efficiently removing PFASs from water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


