西维美林治疗Sjögren综合征患者口干的疗效:随机临床试验的系统回顾和荟萃分析。
IF 1.5
Q3 MEDICINE, RESEARCH & EXPERIMENTAL
Current Therapeutic Research-clinical and Experimental
Pub Date : 2025-01-01
DOI:10.1016/j.curtheres.2024.100770
引用次数: 0
摘要
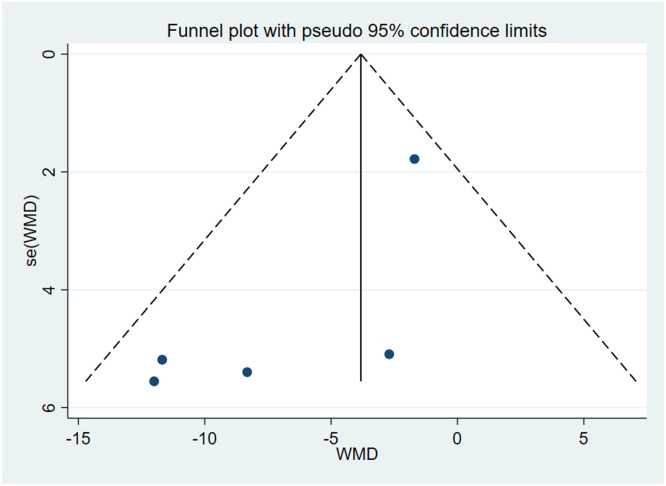
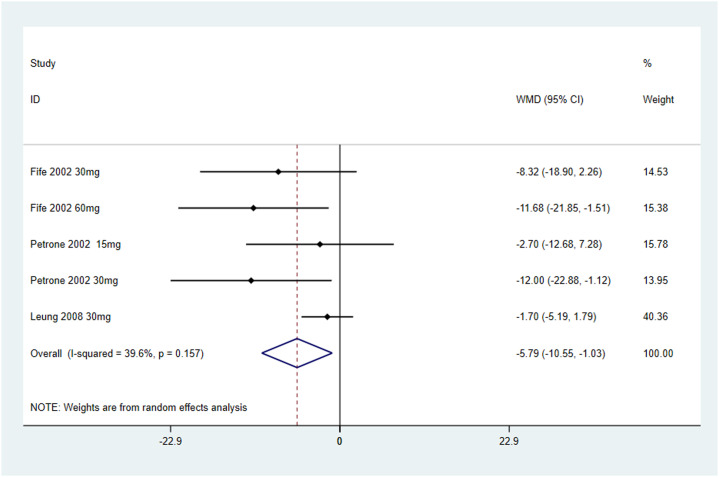
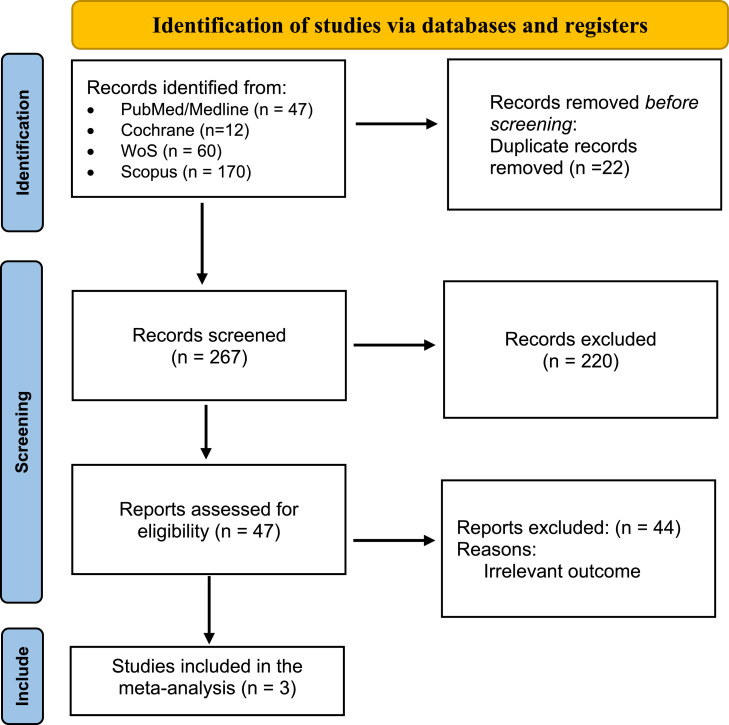
背景:口干,或口干,是Sjögren综合征患者常见的衰弱症状,影响他们的生活质量。虽然西维美林,一种毒蕈碱激动剂,已经作为一种潜在的治疗方法进行了研究,但其疗效和最佳剂量仍然不确定。本研究旨在通过随机临床试验(RCT)的荟萃分析来评估西维美林缓解Sjögren综合征患者口干症的有效性。方法:综合检索PubMed、Scopus、Cochrane和Web of Science数据库,利用医学主题词和关键词“西维美林”、“口干症”和“Sjögren’s syndrome”,从研究开始到2024年1月3日。研究是根据预先确定的纳入标准选择的,重点是涉及使用西维美林治疗Sjögren综合征口干症的人类受试者的临床试验。系统提取数据,使用STATA软件进行统计分析。结果:本荟萃分析纳入3项随机对照试验,共纳入302例Sjögren综合征患者(塞维米林 = 187;安慰剂 = 115)。分析表明,西维美林可显著减少Sjögren综合征患者的口干症(视为唾液流、口干),合并优势比为-5.79 (95% CI [-10.55, -1.03];I 2 = 39.6%)。结论:综上所述,西维美林可显著提高Sjögren综合征患者的唾液分泌率。在推荐剂量下,西维美林具有良好的安全性,是治疗口干症的可行治疗选择,特别是在轻度至中度唾液腺破坏的患者中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Efficacy of Cevimeline on Xerostomia in Sjögren's Syndrome Patients: A Systematic Review and Meta-Analysis of Randomized Clinical Trials
Background
Xerostomia, or dry mouth, is a common and debilitating symptom in patients with Sjögren's syndrome, affecting their quality of life. Although Cevimeline, a muscarinic agonist, has been investigated as a potential treatment, its efficacy and optimal dosage remain uncertain. This study aims to assess the effectiveness of Cevimeline in relieving xerostomia in patients with Sjögren's syndrome by a meta-analysis of randomized clinical trials (RCT).
Method
A comprehensive search was conducted across PubMed, Scopus, Cochrane, and Web of Science databases, utilizing Medical Subject Headings terms and keywords related to “cevimeline,” “xerostomia,” and “Sjögren's syndrome” from inception until January 3, 2024. Studies were selected based on predefined inclusion criteria, focusing on clinical trials involving human subjects treated with cevimeline for xerostomia in Sjögren's syndrome. Data extraction was performed systematically, and statistical analysis was conducted using STATA software.
Result
This meta-analysis included three RCTs with a total of 302 patients with Sjögren's syndrome (Cevimeline = 187; Placebo = 115). The analysis demonstrated that Cevimeline significantly reduces xerostomia (regarded as salivary flow, mouth dryness) in patients with Sjögren's syndrome with a pooled odds ratio –5.79 (95% CI [–10.55, –1.03]; I2 = 39.6%).
Conclusions
In summary, cevimeline significantly increases salivary flow secretion rates in patients with Sjögren's syndrome. With a favorable safety profile at recommended dosages, cevimeline represents a viable therapeutic option for managing xerostomia, particularly in patients with mild to moderate salivary gland destruction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
0.00%
发文量
31
审稿时长
3 months
期刊介绍:
We also encourage the submission of manuscripts presenting preclinical and very preliminary research that may stimulate further investigation of potentially relevant findings, as well as in-depth review articles on specific therapies or disease states, and applied health delivery or pharmacoeconomics.
CTR encourages and supports the submission of manuscripts describing:
• Interventions designed to understand or improve human health, disease treatment or disease prevention;
• Studies that focus on problems that are uncommon in resource-rich countries;
• Research that is "under-published" because of limited access to monetary resources such as English language support and Open Access fees (CTR offers deeply discounted English language editing);
• Republication of articles previously published in non-English journals (eg, evidence-based guidelines) which could be useful if translated into English;
• Preclinical and clinical product development studies that are not pursued for further investigation based upon early phase results.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


