IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
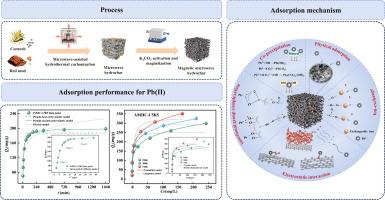
本研究通过微波辅助水热碳化(MHTC)和 K2CO3 活化法成功制备了以低成本玉米芯(CC)和赤泥(RM)为原料的赤泥改性生物炭(AMHC)。创新性地研究了赤泥和 K2CO3 活化对 AMHC 铅(Pb)(II)特性的影响。结果表明,添加 RM 能明显改善 AMHC 的特性,当 CC 与 RM 的质量比为 8:2 时,AMHC 的特殊比表面积最大,为 591.91 m2/g,总孔体积最大,为 0.49 cm3/g。当 CC 与 RM 的质量比为 5:5 时(AMHC-C5R5),AMHC 的磁性最强,为 30.85 emu/g,这有利于通过磁分离将它们从水溶液中分离出来。此外,批量吸附实验的结果还表明,AMHC-C5R5 在 45 ℃ 时的最大吸附容量为 350.28 mg/g,这一过程主要以化学吸附为主。有趣的是,对于未添加 RM 的 AMHC,当铅(II)的初始浓度超过 200 mg/L 时,吸附过程是不自发的,而对于 AMHC-C5R5,直到铅(II)的初始浓度达到 500 mg/L 时,吸附过程仍然是自发的。此外,研究结果还表明,AMHC 表面的-COOH、-OH、Fe3O4 和 Fe2O3 能促进铅(II)与 AMHC 之间的静电吸附。吸附机理不仅包括离子交换和静电作用,还包括沉淀、络合和孔隙填充。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced adsorption for aqueous lead (Ⅱ) by red mud-modified biochar via microwave-assisted hydrothermal carbonization and K2CO3 activation: Performance and mechanism
In this study, red mud-modified biochar (AMHC) derived from low-cost corncob (CC) and red mud (RM) was successfully prepared by microwave-assisted hydrothermal carbonization (MHTC) and K2CO3 activation. The effect of RM and K2CO3 activation on the characteristics of AMHC for lead (Pb) (II) was innovatively investigated. The results showed that adding RM could promote the characteristics of AMHC obviously, and when the mass ratio of CC to RM was 8:2, AMHC exhibited the highest special surface area of 591.91 m2/g and the highest total pore volume of 0.49 cm3/g. And when the mass ratio of CC to RM was 5:5 (AMHC-C5R5), AMHC exhibited the highest magnetic strength of 30.85 emu/g, which was beneficial for separating of them from aqueous solutions by magnetic separation. Moreover, the results of batch adsorption experiment also showed that AMHC-C5R5 exhibited a maximum adsorption capacity of 350.28 mg/g at 45 °C, and this process was dominated by chemisorption. Interestingly, for AMHC without added RM, the adsorption process was non-spontaneous when the initial concentration of Pb (II) exceeded 200 mg/L, while for AMHC-C5R5, the adsorption was still spontaneous until the initial concentration of Pb (II) reach 500 mg/L. Furthermore, the results also indicated that –COOH, –OH, Fe3O4 and Fe2O3 on the surface of AMHC could promote electrostatic adsorption between Pb (II) and AMHC. And the adsorption mechanisms included not only ion exchange and electrostatic interaction, but also precipitation, complexation, and pore filling as well.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


