铜催化末端烯烃丙基化制备跳过烯烃
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
首次报道了一种在铜催化下高效且易于操作的合成跳炔醛的方法。在温和的条件下,以非活化的末端烯烃为亲核物,以可接受到良好的收率获得了多种跳炔醛。该方案具有底物范围广、官能团耐受性高的特点,因此可以进行后期官能化和放大实验,进一步凸显了其合成实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
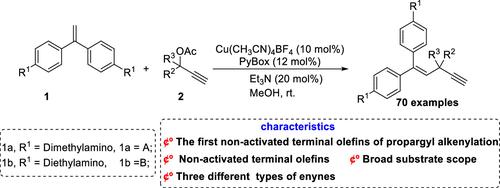
Copper-Catalyzed Propargylation of Terminal Olefins to Skipped Enynes
A highly efficient and easy to operate method for synthesizing skipped enynes catalyzed by copper has been reported for the first time. A wide variety of skipped enynes were obtained in acceptable to good yields employing nonactivated terminal olefins as nucleophiles under mild conditions. This protocol features broad substrate scope and high functional group tolerance, therefore enabling late-stage functionalization and a scale-up experiment further to highlight the synthetic utility.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


