来自上皮细胞的工程化免疫调节细胞外囊泡有能力刺激癌症和自身免疫中的先天性免疫和适应性免疫
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
所有细胞都会产生细胞外囊泡 (EV)。尽管上皮细胞和间充质细胞衍生的异源EVs携带有一系列功能分子,包括数千种蛋白质,但其全身给药已被证明是安全的。为了解决上皮细胞衍生的 EV 是否能被改造以获得诱导免疫反应的能力,我们改造了 293T EV,使其携带免疫调节分子 CD80、OX40L 和 PD-L1。我们在工程化细胞和 EVs 中发现了大量这些蛋白。在功能上,工程化的 EVs 能有效激发人类和小鼠 T 细胞的正性和负性成本刺激。在癌症和自身免疫性肝炎的情况下,工程EV可调节T细胞功能并改变疾病进展。在黑色素瘤小鼠体内,OX40L EV 与抗 CTLA-4 结合使用还能增强抗肿瘤活性。此外,我们还在 EVs(EVmIM)中添加了多种免疫调节蛋白,试图在淋巴细胞和骨髓细胞中引起免疫反应。含有CD80、4-1BBL、CD40L、CD2和CD32的EVmIM与黑色素瘤肿瘤中的T细胞和抗原呈递细胞(APC)都发生了作用,证明了EVmIM具有激发抗肿瘤活性的能力。我们的工作提供了证据,证明可以通过设计诱导特异性免疫反应的 EV,从而具有在病理环境中调节免疫细胞功能的转化潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
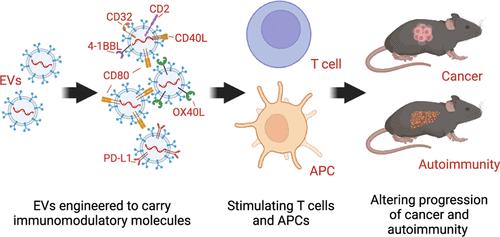
Engineered Immunomodulatory Extracellular Vesicles from Epithelial Cells with the Capacity for Stimulation of Innate and Adaptive Immunity in Cancer and Autoimmunity
Extracellular vesicles (EVs) are generated in all cells. Systemic administration of allogenic EVs derived from epithelial and mesenchymal cells has been shown to be safe, despite carrying an array of functional molecules, including thousands of proteins. To address whether epithelial cell-derived EVs can be modified to acquire the capacity to induce an immune response, we engineered 293T EVs to harbor the immunomodulatory molecules CD80, OX40L, and PD-L1. We demonstrated abundant levels of these proteins in the engineered cells and EVs. Functionally, the engineered EVs efficiently elicited positive and negative costimulation of human and murine T cells. In the setting of cancer and autoimmune hepatitis, the engineered EVs modulated T cell functions and altered disease progression. OX40L EVs also provided enhanced antitumor activity in combination with anti-CTLA-4 in melanoma-bearing mice. In addition, we added multiple immunomodulatory proteins in EVs (EVmIM), attempting to elicit an immune response in both lymphoid and myeloid compartments. The EVmIM containing CD80, 4-1BBL, CD40L, CD2, and CD32 engaged both T cells and antigen presenting cells (APCs) in melanoma tumors, demonstrating the capacity for EVmIM to elicit antitumor activity. Our work provides evidence that EVs can be engineered to induce specific immune responses with translational potential to modulate immune cell functions in pathological settings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


