一种用于活细胞乙酰辅酶A可视化的基因编码荧光生物传感器
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
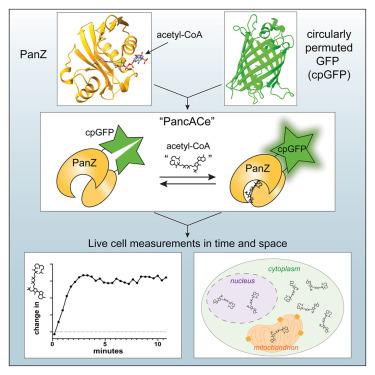
乙酰辅酶A是参与许多细胞途径的中心代谢物。有证据表明,在哺乳动物细胞中,乙酰辅酶a代谢是高度区隔化的。然而,目前还缺乏测量活细胞中乙酰辅酶a的方法。在此,我们利用细菌蛋白PanZ和循环排列绿色荧光蛋白(cpGFP)设计了乙酰辅酶a生物传感器。该传感器“PancACe”的最大变化为~ 2倍,响应范围为~ 10 μM-2 mM乙酰辅酶a。我们证明了该传感器对辅酶a、丁基辅酶a、丙二酰辅酶a和琥珀酰辅酶a的选择性大于7倍,对丙酰辅酶a的选择性大于2.3倍。我们在大肠杆菌中表达了该传感器,并表明它能够检测乙酰辅酶a水平的快速变化。通过将传感器定位到人类细胞的细胞质、细胞核或线粒体,我们发现它可以亚细胞检测乙酰辅酶a水平的变化,其幅度与正交PicoProbe测定一致。本文章由计算机程序翻译,如有差异,请以英文原文为准。

A genetically encoded fluorescent biosensor for visualization of acetyl-CoA in live cells
Acetyl-coenzyme A is a central metabolite that participates in many cellular pathways. Evidence suggests that acetyl-CoA metabolism is highly compartmentalized in mammalian cells. Yet methods to measure acetyl-CoA in living cells are lacking. Herein, we engineered an acetyl-CoA biosensor from the bacterial protein PanZ and circularly permuted green fluorescent protein (cpGFP). The sensor, “PancACe,” has a maximum change of ∼2-fold and a response range of ∼10 μM–2 mM acetyl-CoA. We demonstrated that the sensor has a greater than 7-fold selectivity over coenzyme A, butyryl-CoA, malonyl-CoA, and succinyl-CoA, and a 2.3-fold selectivity over propionyl-CoA. We expressed the sensor in E. coli and showed that it enables detection of rapid changes in acetyl-CoA levels. By localizing the sensor to either the cytoplasm, nucleus, or mitochondria in human cells, we showed that it enables subcellular detection of changes in acetyl-CoA levels, the magnitudes of which agreed with an orthogonal PicoProbe assay.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


