质子化依赖性电荷跨二苯基胺单分子结的传输
IF 4.8
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
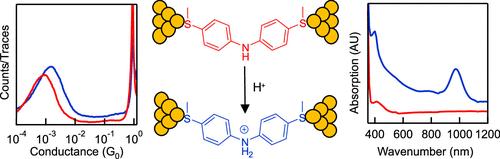
Protonation-Independent Charge Transport Across Diphenylamine Single-Molecule Junctions
Amines are one of the most ubiquitous functional groups in molecular junctions; however, the exact regulation of the charge transport through the protonation state of an amine group in the junction backbone remains elusive. We address this question here by designing a diphenylamine molecular backbone and experimentally investigating how protonation of the central amine group affects the charge transport. Our ultraviolet–visible spectroscopy measurements demonstrate the protonation reaction of the diphenylamine compound in the presence of either trifluoroacetic acid or HCl, and we observe a consistent trend of a modestly increased conductance for diphenylamine in the presence of acid, indicating that a protonated amine group in a diphenylamine backbone slightly enhances the electron conduction. We further investigate the charge transport across diphenylamine under a series of applied tip bias voltages between −0.9 to 0.9 V in an electrochemical environment in the absence and presence of acid for determining the frontier molecular orbital alignment with the Fermi level and the coupling coefficient between the molecule and the electrodes. Our finding shows that the highest occupied molecular orbital (HOMO) is the dominating transport channel of the diphenylamine junction, and a modest conductance increase is an outcome of the HOMO resonance energy moving closer to the Fermi level upon protonation of the amine.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


