II型MIL-100/BiOBr异质结促进了盐酸四环素的高效光- fenton降解
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
由于铁基金属有机框架(MOFs)中光生载流子分离效率低、Fe2+/Fe3+循环缓慢,限制了其光-芬顿活性的提高。因此,本研究通过简单的溶剂蒸发法将 MIL-100 与 BiOBr 纳米片复合在一起,构建了 MIL-100/BiOBr 异相光-芬顿催化剂。在该体系中,MIL-100 与 BiOBr 紧密接触形成的 II 型异质结可以促进光生载流子的分离,加速 Fe2+/Fe3+ 的氧化还原循环,从而显著提高光-芬顿活性。在可见光照射 60 分钟的条件下,MIL-100/BiOBr 对盐酸四环素(TCH)的最佳去除率为 94.3%。值得注意的是,在光-芬顿体系中,MIL-100/BiOBr 的降解速率常数比 MIL-100 提高了近四倍。此外,催化剂还能在较宽的 pH 值范围(3-9)和不同阴离子共存的情况下保持较高的降解性能。对降解机理的深入研究表明,超氧自由基(-O2-)和羟自由基(-OH)在光-芬顿体系中发挥了主要作用。在此基础上,提出了可能的降解途径。因此,本研究为铁基 MOFs 通过光-芬顿反应去除废水中的新兴污染物提供了一种新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
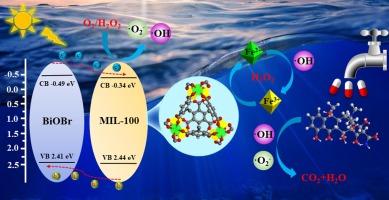
The type II MIL-100/BiOBr heterojunctions promote efficient photo-Fenton degradation of tetracycline hydrochloride
The low efficiency of photogenerated carriers separation and slow cycling of Fe2+/Fe3+ in Fe-based metal–organic frameworks (MOFs) have limited the improvement of their photo-Fenton activity. Therefore, in this work, MIL-100/BiOBr heterogeneous photo-Fenton catalyst was constructed by compositing MIL-100 with BiOBr nanosheets through a simple solvent evaporation method. In this system, the type II heterojunctions formed by the close contact of MIL-100 and BiOBr can promote the separation of photogenerated carriers and accelerate the redox cyclic of Fe2+/Fe3+, thereby significantly enhancing the photo-Fenton activity. Under visible light irradiation for 60 min, the best removal rate of tetracycline hydrochloride (TCH) by MIL-100/BiOBr is 94.3 %. Remarkably, the MIL-100/BiOBr exhibits a nearly fourfold increase in degradation rate constant compared to MIL-100 at photo-Fenton system. In addition, the catalysts can maintain high degradation performance over a wide pH range (3–9) and the co-existence of different anions. An in-depth investigation of the degradation mechanism reveals that superoxide radicals (·O2–) and hydroxyl radicals (·OH) play major roles in the photo-Fenton system. Based on this, the possible degradation pathways were proposed. Therefore, this study provides a new strategy for Fe-based MOFs to remove emerging pollutants from wastewater by photo-Fenton reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


