频繁攻击者E3连接酶在靶向蛋白降解筛选中的意义
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
靶向蛋白降解(Targeted protein degradation, TPD)为化学探针和药物发现提供了一种很有前途的方法,它利用小分子或生物制剂将蛋白质引导到细胞机制中进行破坏。在600种人类E3连接酶中,CRBN和VHL是泛素-蛋白酶体系统依赖性TPD的主要途径。确定能够支持TPD的其他E3连接酶将释放该机制在研究和制药应用中的全部潜力。这一观点讨论了最近扩大TPD范围的策略,以及这些不同筛选工作在少数E3连接酶(特别是DCAF16、DCAF11和FBXO22)上的惊人融合。我们推测,包括表面配体性、潜在的混杂底物相互作用和Cullin-RING复合物的高占用率在内的一系列特性,可能使这些E3连接酶在TPD筛选中成为“低垂的果实”。我们还讨论了可能进一步扩展支持TPD的E3连接酶领域的互补方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。

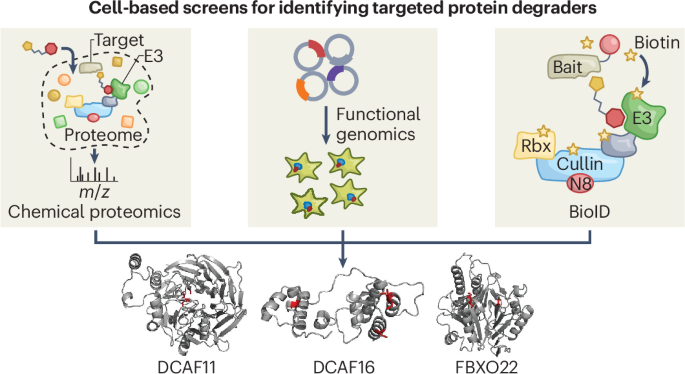
Implications of frequent hitter E3 ligases in targeted protein degradation screens
Targeted protein degradation (TPD) offers a promising approach for chemical probe and drug discovery that uses small molecules or biologics to direct proteins to the cellular machinery for destruction. Among the >600 human E3 ligases, CRBN and VHL have served as workhorses for ubiquitin–proteasome system-dependent TPD. Identification of additional E3 ligases capable of supporting TPD would unlock the full potential of this mechanism for both research and pharmaceutical applications. This perspective discusses recent strategies to expand the scope of TPD and the surprising convergence of these diverse screening efforts on a handful of E3 ligases, specifically DCAF16, DCAF11 and FBXO22. We speculate that a combination of properties, including superficial ligandability, potential for promiscuous substrate interactions and high occupancy in Cullin–RING complexes, may position these E3 ligases as ‘low-hanging fruit’ in TPD screens. We also discuss complementary approaches that might further expand the E3 ligase landscape supporting TPD. This Perspective discusses recent strategies to expand the scope of targeted protein degradation (TPD) and the implications of unexpected convergence of diverse screening efforts on a small subset of TPD-competent E3 ligases in the human proteome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


