通过人类干预试验和分层贝叶斯种群模型推导出替那唑酸的人体毒性动力学参数和毒理学关注的内部阈值。
IF 4.7
3区 医学
Q2 ENVIRONMENTAL SCIENCES
Journal of Exposure Science and Environmental Epidemiology
Pub Date : 2025-01-24
DOI:10.1038/s41370-025-00746-6
引用次数: 0
摘要
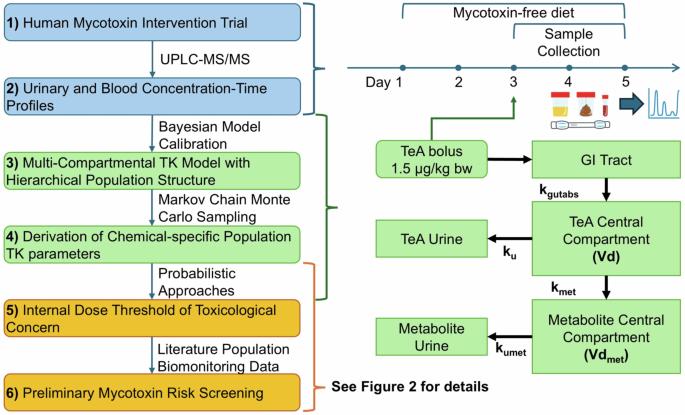
背景:Tenuazonic acid (TeA)是一种由交替孢霉产生的霉菌毒素,可污染各种食品,已知可引起急性和慢性健康影响。然而,由于缺乏人体毒物动力学(TK)数据和依赖外部暴露估计,对TeA的全面风险评估停滞不前。目的:为了弥补这一空白,采用人类TK试验和基于人群的TK (PopTK)模型来确定TeA的人类TK参数,并将结果应用于基于人群生物监测数据和基于毒性关注阈值(TTC)的方法进行风险筛查。方法:10名健康志愿者参加TK试验,在试验期间,志愿者以(外)TTC (1500 ng/kg bw)的剂量摄入茶。在48小时内采集血液、尿液和粪便样本,使用UPLC-MS/MS进行分析。浓度-时间分布曲线采用分层贝叶斯种群结构的多分区PopTK模型拟合。利用概率框架,拟合的TK参数用于获得内部TTC (iTTC)值,以便与血液和尿液生物监测数据进行比较。使用危险商(HQ)和概率个体暴露边际(IMOE)方法对来自五个不同生物监测队列的数据进行风险筛查。结果:估计TeA的群体中位半衰期为1.9 [90% CI: 1.4-2.7]小时,分布体积为4.4 [3.1-6.1]L/kg,个体间变异几何标准差分别为2.4倍和1.7倍。概率较低置信下限ittc分别为0.5 nmol/L血液和2.53 nmol/kg-d尿液排泄。3个血液生物监测队列的风险筛查hq大多为bb0.1, 2个尿液生物监测队列的风险筛查hq大多为< 1;概率IMOE计算结果在质量上是一致的。意义:首次对TeA进行了全面的人类传统知识研究,证明了将传统知识和种群变异结合起来进行更全面的风险评估的重要性,特别是在解释生物监测数据方面。TeA的结果表明,除了基于ttc的风险筛查之外,还迫切需要毒性数据。影响:解决了食品安全研究中的一个关键空白,即研究人体中tenuazonic acid (TeA)的毒性动力学,并利用这些数据得出毒理学关注的内部阈值(iTTC),以便与人体生物监测数据进行比较。这种创新的方法——将人类干预试验与基于人群的毒物动力学模型相结合——解释了个体间的差异,并提供了对人群暴露于TeA的更全面的理解。由此产生的概率iTTC和风险筛选方法为解释生物监测数据提供了改进的工具。这些发现对食品安全法规和公共卫生保护具有重要意义,可能影响未来的霉菌毒素风险评估策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Derivation of human toxicokinetic parameters and internal threshold of toxicological concern for tenuazonic acid through a human intervention trial and hierarchical Bayesian population modeling
Tenuazonic acid (TeA), a mycotoxin produced by Alternaria alternata, contaminates various food commodities and is known to cause acute and chronic health effects. However, the lack of human toxicokinetic (TK) data and the reliance on external exposure estimates have stalled a comprehensive risk assessment for TeA. To bridge this gap, a human TK trial and population-based TK (PopTK) modeling were applied to determine human TK parameters of TeA, and the results were applied for risk screening using population biomonitoring data and threshold of toxicological concern (TTC)-based approaches. Ten healthy volunteers participated in the TK trial during which the volunteers ingested a bolus dose of TeA at the (external) TTC (1500 ng/kg bw). Blood, urine, and fecal samples were collected over 48 h and analyzed using UPLC-MS/MS. Concentration-time profiles were fit with a multi-compartmental PopTK model using a hierarchical Bayesian population structure. Utilizing a probabilistic framework, fitted TK parameters were used to derive internal TTC (iTTC) values for comparison to blood and urine biomonitoring data. Risk screening with data from five diverse biomonitoring cohorts was performed using Hazard Quotient (HQ) and probabilistic individual margin of exposure (IMOE) approaches. TeA was estimated to have a population median half-life of 1.9 [90% CI: 1.4–2.7] hours and volume of distribution of 4.4 [3.1–6.1] L/kg, with inter-individual variability geometric standard deviations of 2.4- and 1.7-fold, respectively. Probabilistic lower confidence bound iTTCs were derived of 0.5 nmol/L in blood and 2.53 nmol/kg-d urinary excretion. Risk screening HQs were mostly >1 for the three blood biomonitoring cohorts and < 1 for the two urinary biomonitoring cohorts; results from probabilistic IMOE calculations were qualitatively consistent. A comprehensive human TK study was performed for TeA for the first time, demonstrating the importance of integrating TK and population variability for a more comprehensive risk evaluation, particularly for interpreting biomonitoring data. The results for TeA point to the critical need for toxicity data to move beyond TTC-based risk screening.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.90
自引率
6.70%
发文量
93
审稿时长
3 months
期刊介绍:
Journal of Exposure Science and Environmental Epidemiology (JESEE) aims to be the premier and authoritative source of information on advances in exposure science for professionals in a wide range of environmental and public health disciplines.
JESEE publishes original peer-reviewed research presenting significant advances in exposure science and exposure analysis, including development and application of the latest technologies for measuring exposures, and innovative computational approaches for translating novel data streams to characterize and predict exposures. The types of papers published in the research section of JESEE are original research articles, translation studies, and correspondence. Reported results should further understanding of the relationship between environmental exposure and human health, describe evaluated novel exposure science tools, or demonstrate potential of exposure science to enable decisions and actions that promote and protect human health.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


