表面活性剂诱导含铬schwertmanite转变和铬酸盐在溶解和再结晶过程中的重分布机理研究
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
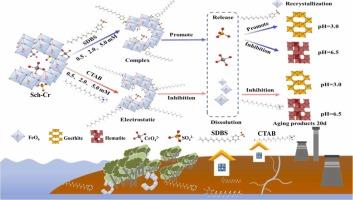
施魏锰矿(Schwertmannite, Sch)是一种典型的亚稳羟基硫酸铁矿物,存在于酸性硫酸盐土壤和矿山排水中,易受环境因素的影响,易被困重金属转化并随之释放和再吸附。因此,了解诱导重金属转化和控制重金属再分布的机制对环境科学和地球化学研究都是不可或缺的。鉴于表面活性剂通过人类活动在环境中广泛积累,一个重要但尚未得到充分研究的问题出现了:在这种环境条件下,表面活性剂是如何影响Sch的转化的?为了回答这个问题,本研究研究了阴离子和阳离子表面活性剂诱导含铬(Cr) schwertmannite (Sch-Cr)的转变以及随后Cr(VI)的再分配。表面活性剂类型和pH值对矿物的转化均有影响。具体来说,在pH为3.0时,最终产物为针铁矿,其中阴离子和阳离子表面活性剂分别促进和抑制矿物转化。在pH 6.5时,赤铁矿是最终的转化产物,阴离子表面活性剂和阳离子表面活性剂都抑制了矿物的转化。对于初始固定的Cr(VI),在十二烷基苯磺酸钠(SDBS)存在下时效20 d后,Cr(VI)主要以溶液形式存在,而在西曲溴铵(CTAB)存在下,Cr(VI)主要以新形成的次生矿物形式存在。本研究揭示了酸性硫酸盐土壤中Sch在环境扰动下的复杂地球化学行为,为Sch作为重金属吸附剂的利用和开发潜力提供了新的思路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mechanistic Insights into Surfactant-Induced Transformation of Chromium-bearing Schwertmannite and Chromate Redistribution during Dissolution and Recrystallization
Schwertmannite (Sch) is a typical metastable iron hydroxysulfate mineral found in acid sulfate soil and mine drainage, and it is susceptible to environmental factors and the transformation and concomitant release and re-adsorption of trapped heavy metals. Therefore, understanding the mechanism that induces Sch transformation and governs heavy metal redistributions is integral for both environmental science and geochemistry research. Given the widespread environmental accumulation of surfactants through human activities, an important yet understudied question arises: How do surfactants influence the transformation of Sch under such environmental conditions? To answer this query, this study investigated the transformation of chromium (Cr)-bearing schwertmannite (Sch-Cr) induced by anionic and cationic surfactants and the subsequent redistribution of Cr(VI). Both the surfactant type and pH affected the transformation of minerals. Specifically, at pH 3.0, goethite was the final product, in which the anionic and cationic surfactant promoted and inhibited mineral transformation, respectively. At pH 6.5, hematite was the final transformation product and both anionic and cationic surfactants inhibited the transformation of minerals. For the initial fixed Cr(VI), after 20 d of aging in the presence of sodium dodecylbenzesulfonate (SDBS), Cr(VI) mainly existed in the form of a solution, whereas in the presence of cetrimonium bromide (CTAB), Cr(VI) mainly existed in the newly formed secondary minerals. This study reveals the complex geochemical behavior of Sch under environmental disturbances in acid sulfate soils and provides insights into the use and potential exploitation of Sch as an adsorbent for heavy metal elements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


