共面噻唑-噻唑金属-有机骨架中空间间价电荷转移的量化及其光致变色行为
IF 4.6
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
分子组装中单个氧化还原单元之间的电子耦合决定了它们的电荷转移效率。金属有机骨架(MOF)是一种定义良好的晶体结构,确保了氧化还原活性基团的正确定位,并为揭示其电荷转移动力学和定量结构关系提供了独特的平台。在这里,我们通过在光或电化学还原的氧化还原活性噻唑基配体(DPTTZ)中引入混合价态,展示了一种具有近红外空间价间电荷转移的新型氧化还原活性MOF。与裸配体体系(4 Å)相比,具有氧化还原活性的DPTTZ分子之间的分离更小(3.75 Å),从而导致更高的通过空间的电子耦合,从而使载流子生长和重组更快,并在MOF中具有更高的有效迁移率。利用MOF具有可见颜色变化的光还原能力表现出光致变色行为,并进一步在紫外照射下制作固态光致变色器件作为潜在的自变暗材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
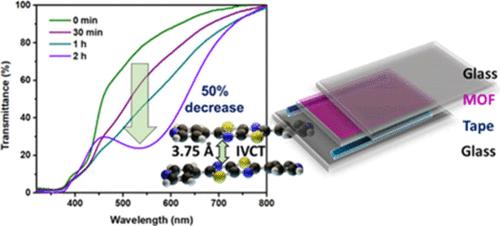
Quantification of Through-Space Intervalence Charge Transfer in Cofacial Thiazolothiazole Metal–Organic Framework and Its Photochromic Behavior
Electronic coupling between individual redox units in a molecular assembly dictates their charge transfer efficacy. Being a well-defined crystalline structure, the metal–organic framework (MOF) ensures proper positioning of redox-active moieties and provides a unique platform to unveil their charge transfer dynamics and quantification with structural relationships. Here, we demonstrate a novel redox-active MOF with near-infrared through-space intervalence charge transfer by introducing a mixed valence state inside redox-active thiazolothiazole-based ligands (DPTTZ) upon photo- or electrochemical reduction. The faster carrier growth and recombination, as well as higher effective mobility in MOF, is attributed to lesser separation between redox-active DPTTZ molecules (3.75 Å) than the bare ligand system (4 Å), which results in higher through-space electronic coupling. The photoreduction ability of the MOF with visible color change was utilized to show photochromic behavior, and further fabrication to a solid-state photochromic device for a potential autodarkening material under UV irradiation was conducted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Physical Chemistry Letters
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
9.60
自引率
7.00%
发文量
1519
审稿时长
1.6 months
期刊介绍:
The Journal of Physical Chemistry (JPC) Letters is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, chemical physicists, physicists, material scientists, and engineers. An important criterion for acceptance is that the paper reports a significant scientific advance and/or physical insight such that rapid publication is essential. Two issues of JPC Letters are published each month.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


