某尾矿库渗流源评价及钼、锌同位素分选。
IF 8
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
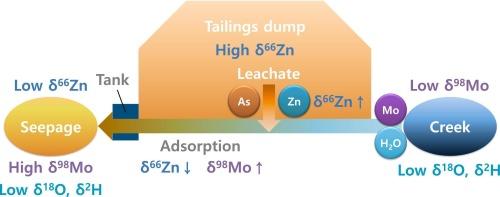
追踪每种污染物的来源及其地球化学反应需要各种各样的地球化学工具。本研究利用O-H、Mo、Zn的化学组成和同位素比值,确定了某矿山尾矿库渗流水、As、Mo、Zn的来源和地球化学反应。渗流与监测井的化学成分差异及O-H同位素比值表明,渗流来源于溪水而非附近地下水,渗流和溪中δ98Mo的季节性变化也支持了这一观点。解释结果表明,钼主要来源于溪中,而砷和锌主要来源于尾矿。在Mo和Zn输运过程中,δ98Mo和δ66Zn分别增加和减少,表明吸附去除,而δ66Zn在尾矿浸出过程中增加。值得注意的是,Mo和Zn同位素比值的联合使用被证明是识别复杂环境系统中地球化学反应和确定来源和途径的有价值的工具。此外,尽管As没有多重同位素,但根据Mo和Zn的同位素行为可以推断出As在Fe(氧)氢氧化物上的可能吸附,因为这两种同位素在吸附过程中有效地反映了同位素分异。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Assessing seepage sources of a tailings dump and fractionation of Mo and Zn isotopes
Tracing the sources of each contaminant and its geochemical reactions requires a variety of geochemical tools. In this study, chemical compositions and isotopic ratios of O-H, Mo, and Zn were utilized to identify the sources and geochemical reactions of water, As, Mo, and Zn in the seepage from a mine tailings dump. The distinct chemical compositions observed between the seepage and monitoring well, along with the O-H isotopic ratios, suggested that the seepage originated from creek water rather than nearby groundwater, which was supported by a large seasonal variation of δ98Mo in both the seepage and creek. Interpretation results indicated that Mo was predominantly supplied from the creek, while the majority of As and Zn originated from the tailings. During the transport of Mo and Zn, δ98Mo and δ66Zn increased and decreased, respectively, suggesting adsorptive removal, despite the δ66Zn increase during the leaching of the tailings. Notably, the combined use of Mo and Zn isotopic ratios proved to be a valuable tool for identifying geochemical reactions and determining sources and pathways in complex environmental systems. Additionally, although As does not have multiple isotopes, possible adsorption of As onto Fe (oxy)hydroxides could be inferred based on the isotopic behavior of Mo and Zn, as these two isotopes effectively reflected isotopic fractionation during adsorption.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science of the Total Environment
环境科学-环境科学
CiteScore
17.60
自引率
10.20%
发文量
8726
审稿时长
2.4 months
期刊介绍:
The Science of the Total Environment is an international journal dedicated to scientific research on the environment and its interaction with humanity. It covers a wide range of disciplines and seeks to publish innovative, hypothesis-driven, and impactful research that explores the entire environment, including the atmosphere, lithosphere, hydrosphere, biosphere, and anthroposphere.
The journal's updated Aims & Scope emphasizes the importance of interdisciplinary environmental research with broad impact. Priority is given to studies that advance fundamental understanding and explore the interconnectedness of multiple environmental spheres. Field studies are preferred, while laboratory experiments must demonstrate significant methodological advancements or mechanistic insights with direct relevance to the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


