脂质-雷帕霉素纳米疫苗在生物治疗中克服了抗药物抗体屏障
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
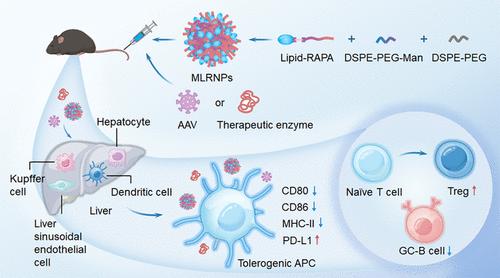
针对生物制剂的抗药物抗体(ADAs)是持续生物治疗的主要挑战,包括酶替代疗法和腺相关病毒(AAV)基因疗法。这些抗体产生于不良的免疫反应,导致药代动力学改变、疗效降低和不良反应。在本研究中,我们引入了一种合理设计的基于脂质-雷帕霉素(Rapa)的纳米疫苗,以恢复对生物制剂的免疫耐受并克服耐药性。当纳米疫苗与锁孔帽贝血青素(KLH)、尿酸酶、聚乙二醇化尿酸酶和AAV8载体基因治疗一起用于耐受性治疗方案时,可显著降低ADA反应。该方法促进了在纳米疫苗5周后对聚乙二醇化尿酸酶的三次再挑战,从而增强了其降低尿酸的功效。此外,纳米疫苗允许成功静脉再给药表达胚胎分泌碱性磷酸酶(AAV8- seap)的AAV8载体,在靶组织中获得持续的病毒DNA和转录物水平。纳米疫苗促使肝脏中的抗原呈递细胞(APCs)表现出CD80、CD86、MHCII和PD-L1的动态变化,这促进了免疫调节性T细胞的发展,以应对生物挑战。值得注意的是,纳米疫苗对CD8+ T细胞、自然杀伤(NK)细胞和NK T细胞的影响最小,保留了机体对病原体和肿瘤的正常免疫反应。总的来说,通用纳米疫苗通过减轻与ada相关的问题来解决生物耐药性问题,从而使抗体、蛋白质和基因疗法的治疗效果得以延长。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Lipid-Rapamycin Nanovaccines Overcome the Antidrug Antibody Barrier in Biologic Therapies
Antidrug antibodies (ADAs) against biologics present a major challenge for sustained biotherapy, including enzyme replacement therapies and adeno-associated virus (AAV) gene therapies. These antibodies arise from undesirable immune responses, leading to altered pharmacokinetics, reduced efficacy, and adverse reactions. In this study, we introduced a rationally designed lipid-rapamycin (Rapa)-based nanovaccine to restore immune tolerance to biologics and overcome drug resistance. The nanovaccine significantly decreased ADA responses when used in a tolerogenic regimen with keyhole limpet hemocyanin (KLH), uricase, pegylated uricase, and AAV8 vector gene therapy. This approach facilitated three rechallenges with pegylated uricase after a 5 week rest from the nanovaccine, thereby enhancing its urate-lowering efficacy. Furthermore, the nanovaccine allowed for the successful intravenous readministration of AAV8 vector expressing secreted embryonic alkaline phosphatase (AAV8-SEAP), achieving sustained viral DNA and transcript levels in target tissues. The nanovaccine prompted antigen-presenting cells (APCs) in the liver to exhibit dynamic changes in CD80, CD86, MHCII, and PD-L1, which promoted the development of immunoregulatory T cells in response to biologic challenges. Notably, the nanovaccine exerted a minimal impact on CD8+ T cells, natural killer (NK) cells, and NK T cells, preserving the body’s normal immune response to pathogens and tumors. Overall, the universal nanovaccine addressed biologic resistance by mitigating ADA-related issues, thereby enabling a prolonged therapeutic efficacy for antibodies, proteins, and gene therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


