金单原子掺杂缺陷纳米多孔铜八面体电催化还原二氧化碳制乙烯
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
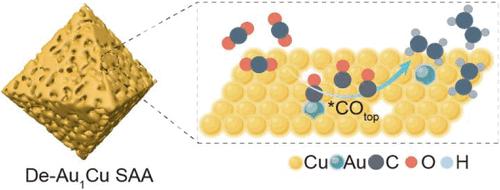
电催化二氧化碳还原为高价值的多碳产品提供了一种可持续的方法,可以关闭人为碳循环,促进碳中和,特别是当使用可再生电力为反应提供动力时。然而,缺乏高效、耐用、对多碳具有高选择性的电催化剂严重阻碍了这项有前途的技术的实际应用。本文通过在氢气中进行低温热还原和随后的脱合金工艺,制备了纳米多孔缺陷Au1Cu单原子合金(De-Au1Cu SAA)催化剂,该催化剂对乙烯(C2H4)具有高选择性,在- 1.1 V电位下,电流密度为252 mA cm-2时,相对可逆氢电极(RHE)的法拉第效率为52%。原位光谱测量和密度泛函理论(DFT)计算表明,C2H4产物的高选择性是由于Au单原子与催化剂表面缺陷Cu位点之间的协同作用,其中Au单原子促进了*CO的生成,Cu缺陷稳定了关键中间体*OCCO,共同增强了C-C耦合动力学。这项工作为电化学CO2还原多碳产物的催化剂设计提供了重要的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Gold Single Atom Doped Defective Nanoporous Copper Octahedrons for Electrocatalytic Reduction of Carbon Dioxide to Ethylene
Electrocatalytic CO2 reduction into high-value multicarbon products offers a sustainable approach to closing the anthropogenic carbon cycle and contributing to carbon neutrality, particularly when renewable electricity is used to power the reaction. However, the lack of efficient and durable electrocatalysts with high selectivity for multicarbons severely hinders the practical application of this promising technology. Herein, a nanoporous defective Au1Cu single-atom alloy (De-Au1Cu SAA) catalyst is developed through facile low-temperature thermal reduction in hydrogen and a subsequent dealloying process, which shows high selectivity toward ethylene (C2H4), with a Faradaic efficiency of 52% at the current density of 252 mA cm–2 under a potential of −1.1 V versus reversible hydrogen electrode (RHE). In situ spectroscopy measurements and density functional theory (DFT) calculations reveal that the high C2H4 product selectivity results from the synergistic effect between Au single atoms and defective Cu sites on the surface of catalysts, where Au single atoms promote *CO generation and Cu defects stabilize the key intermediate *OCCO, which altogether enhances C–C coupling kinetics. This work provides important insights into the catalyst design for electrochemical CO2 reduction to multicarbon products.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


