透明质酸修饰的cuo2 -阿霉素纳米点簇通过三管齐下的策略靶向增敏乳腺癌中的cuprosis。
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
铜质增生在癌症治疗中具有广阔的应用前景。然而,细胞内铜含量不足、低水平的氧化还原稳态和缺氧的肿瘤微环境严重限制了铜增生的疗效。在此,通过三管齐下的策略,开发了肼化透明质酸模板修饰cuo2 -阿霉素(CuDT)纳米点簇(nc),用于有效的阿霉素(DOX)致敏铜细胞沉积治疗乳腺癌。以3,3′-二硫代丙酰肼偶联透明质酸、Cu2+和DOX为原料,通过一锅矿化工艺制备了平均尺寸为56.2 nm的CuDT NCs。CuDT纳米颗粒表现出ph响应的H2O2、Cu2+和DOX释放曲线和催化活性。进入肿瘤细胞后,以cuo2为基础的外源性H2O2供应和dox增强的内源性H2O2产生共同提高细胞内H2O2水平,进而通过fenton样反应转化为羟基自由基和O2,分别达到氧化应激放大和缺氧缓解的目的。此外,CuDT NCs可以通过Cu2+/Cu+循环和丰富的二硫键有效地消耗细胞内过表达的谷胱甘肽,进一步增强细胞氧化应激。这些结果表明,新型CuDT NCs通过提高铜水平、放大氧化应激和缓解缺氧,在乳腺癌细胞中实现dox致敏性铜增生,从而显示出显著的体内抗肿瘤功效。这种三管齐下的策略有针对性地促进癌细胞中的铜增生,代表了一种抗肿瘤治疗的新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
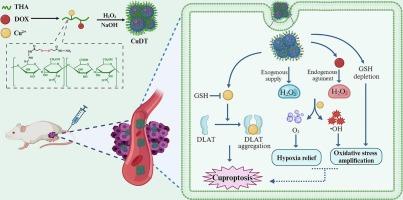
Hyaluronan-decorated CuO2-doxorubicin nanodot clusters for targetedly sensitizing cuproptosis in breast cancer via a three-pronged strategy
Cuproptosis shows great prospects in cancer treatments. However, insufficient intracellular copper amount, low-level redox homeostasis, and hypoxic tumor microenvironment severely restrict cuproptosis efficacy. Herein, hydrazided hyaluronan-templated decorated CuO2-doxorubicin (CuDT) nanodot clusters (NCs) are developed for efficient doxorubicin (DOX)-sensitized cuproptosis therapy in breast cancer via a three-pronged strategy. The CuDT NCs with an average size of 56.2 nm are fabricated from 3,3′-dithiobis(propionohydrazide)-conjugated hyaluronan, Cu2+, and DOX through a one-pot mineralization process. The CuDT nanoparticles exhibit pH-responsive H2O2, Cu2+, and DOX release profiles and catalytic activity. Upon entrance into tumor cells, CuO2-based exogenous H2O2 supply and DOX-augmented endogenous H2O2 generation jointly elevate intracellular H2O2 level, which can further be transformed into hydroxyl radicals and O2 through Fenton-like reaction to achieve oxidative stress amplification and hypoxia relief, respectively. Moreover, the CuDT NCs can efficiently deplete intracellular overexpressed glutathione via Cu2+/Cu+ cycle and abundant disulfide bonds, further enhancing cellular oxidative stress. These results demonstrate that the novel CuDT NCs achieve DOX-sensitized cuproptosis in breast cancer cells through elevating copper level, amplifying oxidative stress and alleviating hypoxia, thus displaying prominent in vivo antitumor efficacy. Such a three-pronged strategy of targetedly boosting cuproptosis in cancer cells represents a novel approach for antitumor treatments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


