钌催化硝基与不饱和羰基化合物的C-C键偶联反应立体选择性合成(Z)-丙烯酸硝基
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
丙烯酸腈是一种多用途的合成前体,用于各种具有药物活性的化合物以及腈聚合物。我们设计了一种由钌催化的腈与不饱和羰基化合物通过C-C键裂解偶联反应立体选择性合成(Z)-丙烯酸腈的方法。碳KIE和Hammett相关数据表明,C-C键裂解是偶联反应的速率决定步骤。采用催化偶联法合成了几种具有生物活性的(Z)-丙烯酸腈。本文章由计算机程序翻译,如有差异,请以英文原文为准。
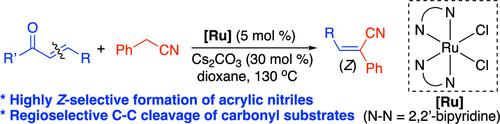
Stereoselective Synthesis of (Z)-Acrylic Nitriles from the Ruthenium-Catalyzed Coupling Reaction of Nitriles with Unsaturated Carbonyl Compounds via C–C Bond Cleavage
Acrylic nitriles are a versatile class of synthetic precursors for a variety of pharmaceutically active compounds, as well as for nitrile polymers. We devised a stereoselective synthesis of (Z)-acrylic nitriles from the Ru-catalyzed coupling reaction of nitriles with unsaturated carbonyl compounds via C–C bond cleavage. Both carbon KIE and Hammett correlation data indicated that C–C bond cleavage is the rate-determining step for the coupling reaction. Several bioactive (Z)-acrylic nitriles were synthesized by using the catalytic coupling method.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


