降解炎性小体的关键成分:NLRP3 PROTAC的开发
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究探索了在先天免疫中起关键作用的NLRP3的PROTACs。我们使用了NLRP3抑制剂MCC950的噻吩类似物,并使用了CuAAC化学来组装带有各种连接体的PROTACs,并招募了三种不同的E3连接酶。化合物通过NLRP3和E3连接酶进行双向热稳定性研究。细胞检测检测IL-1β释放和NLRP3蛋白丰度。PROTAC V2诱导了显著的vhl依赖性NLRP3降解,并构成了破译NLRP3炎性体复杂细节的有价值的工具。本文章由计算机程序翻译,如有差异,请以英文原文为准。
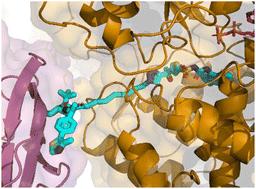
Degrading the key component of the inflammasome: development of an NLRP3 PROTAC
This study explores PROTACs for NLRP3, the key player in innate immunity. We utilised a thiophene analogue of the NLRP3 inhibitor MCC950 and employed CuAAC chemistry for the assembly of PROTACs bearing various linkers and recruiting three different E3 ligases. Compounds were evaluated in bidirectional thermal stability studies with NLRP3 and E3 ligases. IL-1β release and protein abundance of NLRP3 were assessed in cellular assays. PROTAC V2 induces a significant VHL-dependent degradation of NLRP3 and constitutes a valuable tool to decipher the intricate details of the NLRP3 inflammasome.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


