铑/硒双催化从n -磺酰基-1,2,3-三唑中获得2-氨基吡咯
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文报道了一种新的铑/硒双催化工艺,用于n -磺酰基-1,2,3-三唑合成2-氨基吡咯。所提出的协同催化机制包括:Rh(II)催化三唑生成Rh-azavinyl卡苯,然后硒催化生成ylide,随后与另一个Rh-azavinyl卡苯环合。简单温和的双催化策略可容纳多种吸电子和给电子官能团,以中高收率提供各种2-氨基吡咯衍生物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
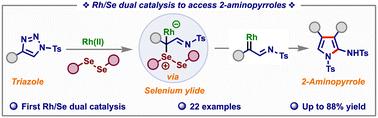
Rhodium/selenium dual catalysis for accessing 2-aminopyrroles from N-sulfonyl-1,2,3-triazoles†
Herein, we report a novel rhodium/selenium dual catalytic process for the synthesis of 2-aminopyrroles from N-sulfonyl-1,2,3-triazoles. The proposed cooperative catalytic mechanism involves Rh(ii)-catalyzed formation of Rh-azavinyl carbene from triazole, followed by selenium-catalyzed generation of ylide, which subsequently undergoes annulation with another Rh-azavinyl carbene. The simple and mild dual catalytic strategy accommodates a variety of electron-withdrawing and electron-donating functional groups, affording various 2-aminopyrrole derivatives in moderate to good yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


