在多药耐药质粒中,KorB从dna滑动钳转换为抑制因子介导远程基因沉默
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
细菌中远程基因调控的例子很少,通常认为与DNA环有关。在这里,我们结合使用生物物理方法,包括x射线晶体学和对大肠杆菌KorB - KorA系统的单分子分析,我们发现质粒RK2上的远程基因沉默是由dna滑动夹(KorB)和夹锁蛋白(KorA)共同实现的,RK2是多种革兰氏阴性菌的多药耐药来源。我们发现KorB是一种CTPase夹子,可以捕获并沿着DNA滑动,到达最远1.5 kb的远端目标启动子。我们解析了KorB - KorA - dna共络合物的三方晶体结构,揭示了KorA将KorB锁存为闭合箝位状态。因此,dna结合的KorA通过阻止KorB在目标启动子处滑动以阻断RNA聚合酶全酶来刺激抑制。总之,我们的发现解释了KorB角色从dna滑动钳转换为协同抑制因子的机制基础,并为细菌中基因表达的远程调控提供了另一种机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

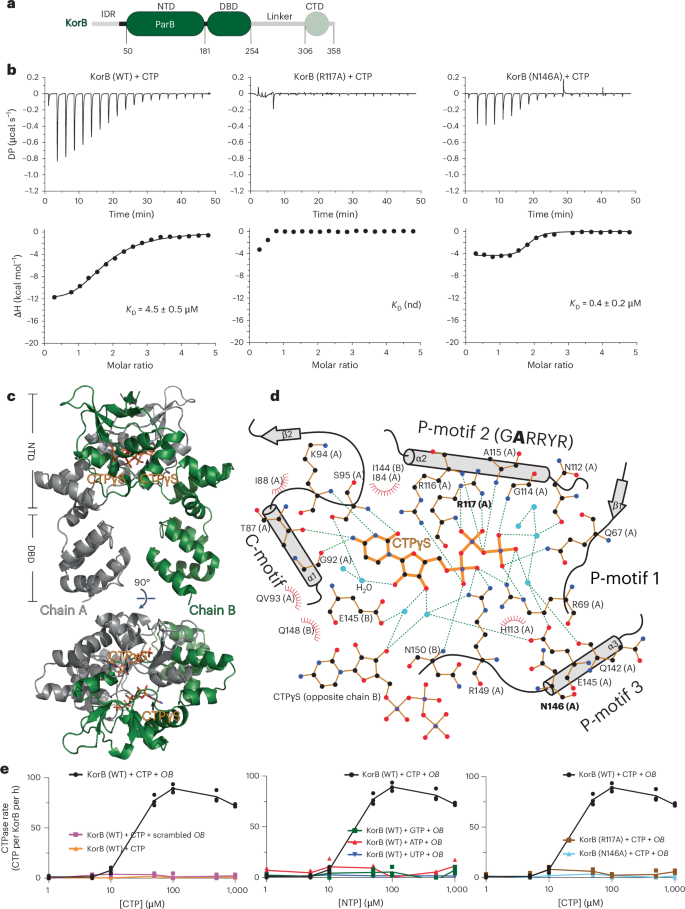
KorB switching from DNA-sliding clamp to repressor mediates long-range gene silencing in a multi-drug resistance plasmid
Examples of long-range gene regulation in bacteria are rare and generally thought to involve DNA looping. Here, using a combination of biophysical approaches including X-ray crystallography and single-molecule analysis for the KorB–KorA system in Escherichia coli, we show that long-range gene silencing on the plasmid RK2, a source of multi-drug resistance across diverse Gram-negative bacteria, is achieved cooperatively by a DNA-sliding clamp, KorB, and a clamp-locking protein, KorA. We show that KorB is a CTPase clamp that can entrap and slide along DNA to reach distal target promoters up to 1.5 kb away. We resolved the tripartite crystal structure of a KorB–KorA–DNA co-complex, revealing that KorA latches KorB into a closed clamp state. DNA-bound KorA thus stimulates repression by stalling KorB sliding at target promoters to occlude RNA polymerase holoenzymes. Together, our findings explain the mechanistic basis for KorB role switching from a DNA-sliding clamp to a co-repressor and provide an alternative mechanism for long-range regulation of gene expression in bacteria. Structural and single-molecule analyses show the CTPase, KorB, is a sliding DNA clamp that interacts with a clamp-locking protein KorA to inhibit gene expression over distances of more than 1 kb in the multi-drug resistance RK2 plasmid.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


