RELMβ设定了微生物组依赖性口服耐受性的阈值
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
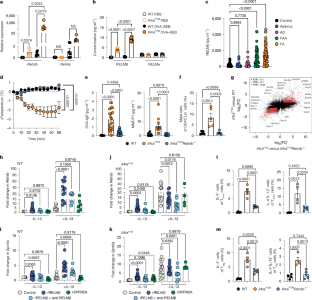
对饮食抗原的耐受性对于避免有害的2型免疫反应导致食物过敏(FA)和过敏反应至关重要1,2。然而,导致对食物抗原的耐受性维持和失败的机制尚不清楚。在这里,我们证明了杯状细胞来源的抵抗素样分子β (RELMβ)3,4是口服耐受性的关键调节因子。RELMβ在FA患者和FA小鼠模型的血清中含量丰富。删除RELMβ可以保护小鼠免受FA和食物抗原特异性IgE的发展和过敏反应。RELMβ通过调节肠道微生物群和消耗产生吲哚代谢物的乳酸菌和乳酸菌来破坏食物耐受性。通过激活芳基烃受体,局部产生吲哚衍生物驱动FA保护性的RORγt+调节性T (Treg)细胞5,从而维持耐受性。断奶前后的RELMβ拮抗可恢复口服耐受性,并保护遗传易感的后代在以后的生活中不患FA。总之,我们表明,RELMβ介导肠道免疫上皮回路调节对食物抗原的耐受性,这是一种通过微生物组编辑先天控制适应性免疫的新模式,并在该回路中确定了预防和治疗FA的靶向候选物。本文章由计算机程序翻译,如有差异,请以英文原文为准。

RELMβ sets the threshold for microbiome-dependent oral tolerance
Tolerance to dietary antigens is critical for avoiding deleterious type 2 immune responses resulting in food allergy (FA) and anaphylaxis1,2. However, the mechanisms resulting in both the maintenance and failure of tolerance to food antigens are poorly understood. Here we demonstrate that the goblet-cell-derived resistin-like molecule β (RELMβ)3,4 is a critical regulator of oral tolerance. RELMβ is abundant in the sera of both patients with FA and mouse models of FA. Deletion of RELMβ protects mice from FA and the development of food-antigen-specific IgE and anaphylaxis. RELMβ disrupts food tolerance through the modulation of the gut microbiome and depletion of indole-metabolite-producing Lactobacilli and Alistipes. Tolerance is maintained by the local production of indole derivatives driving FA protective RORγt+ regulatory T (Treg) cells5 through activation of the aryl hydrocarbon receptor. RELMβ antagonism in the peri-weaning period restores oral tolerance and protects genetically prone offspring from developing FA later in life. Together, we show that RELMβ mediates a gut immune–epithelial circuit regulating tolerance to food antigens—a novel mode of innate control of adaptive immunity through microbiome editing—and identify targetable candidates in this circuit for prevention and treatment of FA. RELMβ mediates a gut immune–epithelial circuit regulating tolerance to food antigens, offering targetable candidates for the prevention and treatment of food allergies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


