一种用于CO2硼氢化催化的c3对称铝酸盐氢化物:机理和反阳离子对催化性能的影响
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
本研究详细介绍了由三齿三酚酸酯配体支撑的坚固单体Al-H铝酸盐的合成和表征,分离为[2][Li(THF)4]和[2][N(nBu)4]盐,然后将其用作CO2氢硼化催化剂。在初始反应性研究中,发现[2][C]中的亲核Al-H阴离子(C =反离子[Li(THF)4]+或[N(nBu)4]+)与CO2反应迅速,生成相应的甲酸al配合物[3][C],并对其进行了分离和结构表征。然后利用这些阴离子作为潜在的二氧化碳还原催化剂。盐[2-3][N(nBu)4]在pinBH或Me2S-BH3作为硼烷源的情况下是高效和稳定的CO2硼氢化催化剂,根据反应条件和反正离子的性质,选择性地产生甲酸当量或甲醇当量的产物(TON up 1920)。从详细的DFT计算中推断,甲酸铝阴离子[3]-作为亲核催化剂(硼烷活化),也作为亲电试剂(通过AlOCO碳),允许CO2活化/功能化,从而发生还原催化,这是一个由还原产物稳定性驱动的热力学过程。[3] -和[3]-铝酸盐的阴离子性质导致其亲核性增强(相对于中性类似物),因此可能对催化活性至关重要。相比之下,根据[3]-和pinBH模型阴离子的DFT计算,通过Al-O / B-H σ-键重分解的CO2还原过程在动力学上是不利的。所提出的机制涉及亲电/亲核双重激活模式,也证明了[2-3][C]中的反阳离子[C]+对催化活性和选择性的重要性,[2][N(nBu)4]比[2][Li(THF)4]的性能更高。本文章由计算机程序翻译,如有差异,请以英文原文为准。
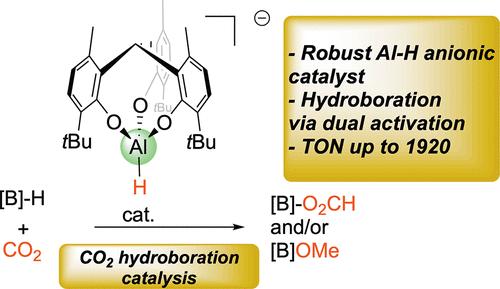
A Robust C3-Symmetric Aluminate Hydride for CO2 Hydroboration Catalysis: Mechanistic Insights and Countercation Influence on Catalytic Performance
The present study details the synthesis and characterization of a robust, monomeric Al–H aluminate supported by a tridentate tris-phenolate ligand, isolated as [2][Li(THF)4] and [2][N(nBu)4] salts, which were then exploited as CO2 hydroboration catalysts. As initial reactivity studies, it was observed that the nucleophilic Al–H anion in [2][C] (C = countercation [Li(THF)4]+ or [N(nBu)4]+) reacts fast with CO2, to afford the corresponding Al-formate complexes [3][C], which were isolated and structurally characterized. Such anions were then exploited as potential CO2 reduction catalysts. Salts [2–3][N(nBu)4] are efficient and robust CO2 hydroboration catalysts in the presence of pinBH or Me2S-BH3 as hydroborane sources to selectively afford formate-equivalent or methanol-equivalent products (TON up 1920), depending on reaction conditions and the nature of the countercation. As deduced from detailed DFT calculations, the Al-formate anion [3]− acts as a nucleophilic catalyst (for borane activation) but also as an electrophile (through the AlOCO carbon) allowing CO2 activation/functionalization and thus the reduction catalysis to occur, a process thermodynamically driven by the stability of the reduction products. The anionic nature of [2]− and [3]− aluminates, resulting in an enhanced nucleophilicity (vs neutral analogues), may thus be crucial for catalytic activity. In contrast, according to DFT calculations performed with a model anion of [3]− and pinBH, a CO2 reduction processing via an Al–O/B–H σ-bond metathesis appears to be kinetically unfavored. The proposed mechanism involving an electrophilic/nucleophilic dual-activation mode also rationalizes the importance of countercation [C]+ in [2-3][C] for catalytic activity and selectivity, as demonstrated by the higher performance of [2][N(nBu)4] vs [2][Li(THF)4].
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


