钼和硫掺杂对Ni-Mo-S电催化剂析氢反应活性位点和d带中心的调控
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
确定活性位点并进一步优化其活性,对于提高析氢反应(HER)性能,特别是对掺杂金属和非金属元素的廉价镍基催化剂具有重要意义。本文报道了掺入钼和硫对提高镍的HER活性的作用。钼和硫共掺杂Ni (NMS)电催化剂表现出优异的HER性能,其过电位和Tafel斜率分别为77.0 mV (10 mA·cm-2)和64.4 mV·dec1,这是因为其电化学活性表面积大、反应动力学快、电荷转移能力强。理论计算结果表明,引入的Mo原子起到了反应位的作用,而引入的S原子可以进一步提高Mo原子的活性。Mo原子的高HER活性可归因于通过优化NMS的组成来调节d波段中心,从而调节Mo与中间体之间的相互作用。这为设计金属和非金属掺杂镍基高效析氢电催化剂提供了可能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
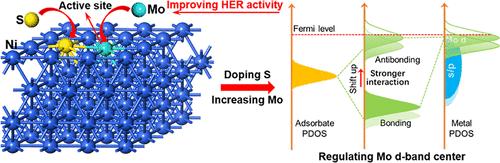
Molybdenum and Sulfur Doping To Create Active Sites and Regulate d-Band Centers of Ni–Mo–S Electrocatalysts for the Hydrogen Evolution Reaction
Defining the active sites and further optimizing their activity are of great significance for enhancing the hydrogen evolution reaction (HER) performances, especially for inexpensive Ni-based catalysts doped with metals and nonmetal elements. This work reports the role of the incorporated molybdenum and sulfur in enhancing the HER activity of nickel. The prepared molybdenum and sulfur coincorporated Ni (NMS) electrocatalysts exhibit excellent HER performance, with an overpotential and Tafel slope of 77.0 mV (10 mA·cm–2) and 64.4 mV·dec–1, respectively, because of the large electrochemically active surface area, quick reaction kinetics, and charge-transfer capability. The theoretical calculation results reveal that the incorporated Mo atoms play the role of reaction sites, and the introduced S atoms can further enhance the activity of Mo atom. The high HER activity of the Mo atom can be attributed to the regulated d-band center by optimizing the composition of NMS, which tunes the interaction between Mo and intermediate species. The elucidated mechanism can make it possible to design Ni-based electrocatalysts doped with metals and nonmetals for efficient hydrogen evolution.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


