层状碲化金(aubte和Au2Te3)的合成及其半导体和金属行为
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
先前对天然镁辉石(AuSbTe)样品的研究揭示了一种潜在的可切割和/或可剥离材料的晶体结构,而对天然和合成月辉石(含sb的Au2Te3)的研究表明,这种低对称性化合物的化学成分多种多样。对这两种材料的合成样品的调查报道很少,留下了许多未知的化学,热学和电子特性。本文研究了金锑碲化物aubte和Au1.9Sb0.46Te2.64(月长石)的稳定性、电子性质和合成。差热分析和原位粉末x射线衍射表明,aubte为不完全熔化,而Au1.9Sb0.46Te2.64为完全熔化。带结构计算和四点电阻率测量表明,aubte为半导体,Au1.9Sb0.46Te2.64为金属。各种合成尝试证实了Au1.9Sb0.46Te2.64有限的稳定化学组成,确定了两种化合物的成功合成方法,并强调了单晶合成AuSbTe的挑战。本文章由计算机程序翻译,如有差异,请以英文原文为准。
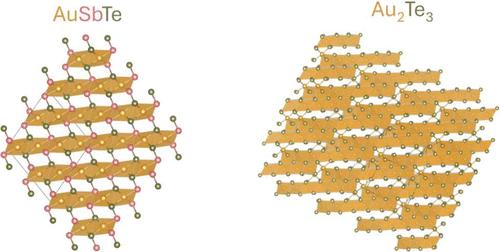
Synthesis of Layered Gold Tellurides AuSbTe and Au2Te3 and Their Semiconducting and Metallic Behavior
Previous studies on natural samples of pampaloite (AuSbTe) revealed the crystal structure of a potentially cleavable and/or exfoliable material, while studies on natural and synthetic montbrayite (Sb-containing Au2Te3) claimed various chemical compositions for this low-symmetry compound. Few investigations of synthetic samples have been reported for both materials, leaving much of their chemical, thermal, and electronic characteristics unknown. Here, we investigate the stability, electronic properties, and synthesis of the gold antimony tellurides AuSbTe and Au1.9Sb0.46Te2.64 (montbrayite). Differential thermal analysis and in situ powder X-ray diffraction revealed that AuSbTe is incongruently melting, while Au1.9Sb0.46Te2.64 is congruently melting. Calculations of the band structures and four-point resistivity measurements showed that AuSbTe is a semiconductor and Au1.9Sb0.46Te2.64 a metal. Various synthesis attempts confirmed the limited stable chemical composition of Au1.9Sb0.46Te2.64, identified successful methods to synthesize both compounds, and highlighted the challenges associated with single-crystal synthesis of AuSbTe.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


