具有拓扑异构酶I和II双重抑制活性的3-芳基异喹啉类化合物的合成、结构修饰及抗小细胞肺癌活性
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
为了克服Topo I和II之间的代偿作用,这是SCLC患者耐药的原因之一,我们正在率先使用3-芳基异喹啉类药物开发Topo I/II的双重抑制剂来治疗SCLC。共合成了46个新化合物。选择化合物3g (NCI-H446细胞IC50 = 1.30 μM, NCI-H1048细胞IC50 = 1.42 μM)和3x (NCI-H446细胞IC50 = 1.32 μM, NCI-H1048细胞IC50 = 2.45 μM)进行详细的药理研究,因为它们具有出色的细胞毒性和双Topo I和II抑制活性。3g和3x通过诱导线粒体凋亡和抑制PI3K/Akt/mTOR通路,有效地阻止SCLC细胞的体外增殖、侵袭和迁移。其体内肿瘤抑制率与依托泊苷相当,毒性更低。这些结果表明它们作为双Topo I和II抑制剂治疗SCLC的潜在治疗价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
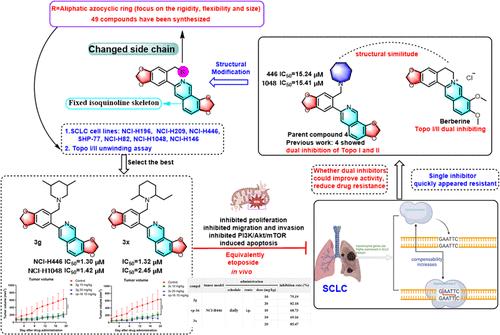
Synthesis, Structural Modification, and Antismall Cell Lung Cancer Activity of 3-Arylisoquinolines with Dual Inhibitory Activity on Topoisomerase I and II
To overcome the compensatory effect between Topo I and II, one of the reasons accounting for the resistance of SCLC patients, we are pioneering the use of 3-arylisoquinolines to develop dual inhibitors of Topo I/II for the management of SCLC. A total of 46 new compounds were synthesized. Compounds 3g (IC50 = 1.30 μM for NCI-H446 cells and 1.42 μM for NCI-H1048 cells) and 3x (IC50 = 1.32 μM for NCI-H446 cells and 2.45 μM for NCI-H1048 cells) were selected for detailed pharmacological investigation, due to their outstanding cytotoxicity and dual Topo I and II inhibitory activity. 3g and 3x effectively prevent SCLC cell proliferation, invasion, and migration in vitro, byinducing mitochondrial apoptosis and inhibiting the PI3K/Akt/mTOR pathway. Their in vivo tumor inhibition rate is comparable to etoposide with lower toxicity. These results indicated their potential therapeutic values as dual Topo I and II inhibitors for treating SCLC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


