非活化烯烃的三组分氯/叠氮-氟烷基化在异相金属光催化剂中的接近效应增强
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
采用可回收催化剂的非均相金属光催化化学转化在有机合成中是非常理想的。然而,为了实现这一目标,合理设计和控制制备定义明确、位置隔离的金属/光双功能多相催化剂仍然是一个重大挑战。在这项研究中,我们证明了一个均相分子MnSalen配合物(其中Salen = N,N ' -双(水杨基)乙二胺)通过酰胺键在石墨氮化碳(CN)表面的共价连接,用于可见光驱动的氯和叠氮氟烷基化未活化的烯烃。MnSalen和CN之间的酰胺共价键不仅促进了电子离域,增强了CN光敏剂的捕光能力,而且在反应过程中还产生了邻近效应,显著提高了Mn位点捕获烷基自由基中间体的能力。多种未活化烯烃可被氯代和叠氮代氟烷基化成相应的双官能团产物,收率中高,官能团相容性好。此外,通过各种生物活性化合物和药物的后期多样化说明了异质协议的实用性。值得注意的是,这种集成光催化剂具有高稳定性,并且可以循环使用至少10次而不损失活性和选择性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
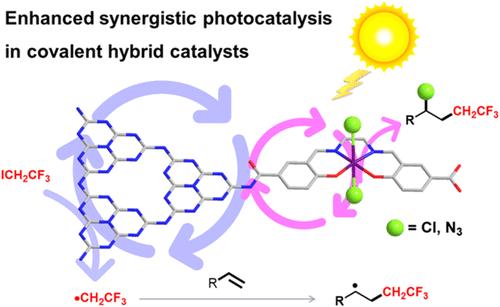
Enhanced Three-Component Chloro-/Azido-Fluoroalkylation of Unactivated Alkenes via the Proximity Effect in a Heterogenous Metallaphotocatalyst
Heterogeneous metallaphotocatalytic chemical transformations employing a recyclable catalyst are highly desirable for organic synthesis. However, the rational design and controlled preparation of well-defined, site-isolated metal/photo bifunctional heterogeneous catalysts to achieve this goal remain a significant challenge. In this study, we demonstrate the covalent attachment of a homogeneous molecular MnSalen complex (where Salen = N,N′-bis(salicylidene)ethylenediamine) onto the surface of graphitic carbon nitride (CN) via an amide bond for visible-light-driven chloro- and azido-fluoroalkylation of unactivated alkenes. The amide covalent linkage between MnSalen and CN not only facilitates electron delocalization and enhances the light-harvesting capabilities of the CN photosensitizer but also exerts a proximity effect that markedly enhances the ability of the Mn sites to capture alkyl radical intermediates during the reaction process. A diverse set of unactivated alkenes could be efficiently chloro- and azido-fluoroalkylated to their corresponding difunctionalized products in moderate to high yields with good functional group compatibility. Furthermore, the practicability of the heterogeneous protocol is illustrated through the late-stage diversification of various bioactive compounds and pharmaceuticals. Notably, this integrated photocatalyst demonstrates high stability and can be recycled at least 10 times without loss of activity and selectivity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


