熔融镍溶液中六方氮化硼的生长:反应分子动力学研究
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
金属助熔剂法是制备高质量六方氮化硼(hBN)晶体的理想方法,但对六方氮化硼在这些体系中成核和生长的原子机制了解甚少,且难以进行实验探索。在这里,我们利用经典反应分子动力学(ReaxFF)揭示了在30 ns的时间尺度上从液态镍溶剂合成hBN的机制。这些模拟模拟了实验条件,包括相对较大的液态镍板,其中含有溶解的硼和分子氮气相。总的来说,由于氮在大块镍中的溶解度低以及中间物质更倾向于金属-气体界面,反应几乎只发生在液态镍的表面。hBN的形成总是由二氮与表面镍溶剂化的硼原子反应开始,形成中间体N-N-B,通常通过一个短寿命的中间体演变成B-N-B单位,其中一个氮原子由一个氮原子和两个硼原子配位。由此产生的B-N-B单元反过来与生长的hBN核结合,并在奥斯特瓦尔德成熟过程中在hBN纳米晶体之间携带氮。在几十纳秒的时间尺度上产生的hBN的数量主要取决于硼的浓度,而对所考虑的N2压力的依赖性要弱得多(N2压力为2.5-10 MPa, Ni-B溶液中硼的原子分数为6-12%)。在考虑的最低温度下(1750 K,刚好高于镍的熔点),hBN的形成速率最高,而在2000 K或更高的温度下没有形成hBN片。对氮原子跃迁途径的分析表明,最后一步,将小的B-N基序结合到较大的hBN片中,是所考虑的制度中的限速步骤。当温度从1750 K升高到2000 K时,对中间产物(N-N-B、B-N - b等)的形成影响不大,而在温度>;1900 K时,没有大的hBN片,这可以解释为最后一步的概率降低,hBN分解成B-N基序的概率增加。本文章由计算机程序翻译,如有差异,请以英文原文为准。
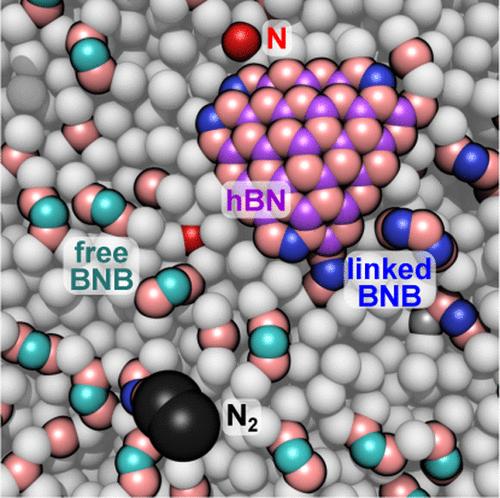
Growth of Hexagonal Boron Nitride from Molten Nickel Solutions: A Reactive Molecular Dynamics Study
Metal flux methods are excellent for synthesizing high-quality hexagonal boron nitride (hBN) crystals, but the atomic mechanisms of hBN nucleation and growth in these systems are poorly understood and difficult to probe experimentally. Here, we harness classical reactive molecular dynamics (ReaxFF) to unravel the mechanisms of hBN synthesis from liquid nickel solvent over time scales up to 30 ns. These simulations mimic experimental conditions by including relatively large liquid nickel slabs containing dissolved boron and a molecular nitrogen gas phase. Overall, the reaction takes place almost exclusively on the surface of the liquid nickel, owing to the low solubility of nitrogen in bulk nickel and the intermediate species’ preference for the metal–gas interface. The formation of hBN invariably begins by reaction of dinitrogen with nickel-solvated boron atoms at the surface, forming intermediate N–N–B species, which typically evolve into B–N–B units through a short-lived intermediate where a single nitrogen atom is coordinated by one nitrogen and two boron atoms. The resulting B–N–B units, in turn, coalesce with growing hBN nuclei and carry nitrogen between hBN nanocrystals in an Ostwald ripening process. The amount of hBN produced on the tens of nanosecond time scale depends critically on the boron concentration, while having a much weaker dependence on the N2 pressure for the regime considered (N2 pressures of 2.5–10 MPa, Ni–B solutions with 6–12% boron by atom fraction). The highest rate of hBN formation occurs at the lowest temperature considered (1750 K, just above the melting point of nickel), while no hBN sheets are formed at 2000 K or above. An analysis of the transition pathways for nitrogen atoms shows that the final step, incorporation of small B–N motifs into larger hBN sheets, is the rate-limiting step in the regimes considered. While raising the temperature from 1750 to 2000 K has little effect on the formation of intermediates (N–N–B, B–N–B, etc.), the lack of large hBN sheets at temperatures >1900 K is explained by decreased probability of the final step and increased probability of breakup of hBN into B–N motifs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


