水基深共晶溶剂一步法直接回收废锂离子电池中的锂
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
锂离子电池(LIB)行业的繁荣与原材料资源(尤其是锂)的稀缺是辩证相关的,因此从废锂中回收关键金属对资源的可持续性至关重要。本文提出了利用水基深度共晶溶剂(DESs)一步法直接从废锂阴极活性材料中回收锂的新策略,并对DESs进行了循环利用。在不需要任何试剂和预处理的情况下,Li2C2O4可直接从H2O基氯化胆碱-草酸(ChCl-OA) DES中优先析出,在富余的富co渗滤液中加入H2O可轻松得到co2o4·2H2O。Li和Co的浸出率接近100%,回收率分别达到91.2%和94.6%。通过Li+与C2O42 -的配位设计,明确了Li和Co的回收机理,实现了Li和Co的精确分离。严格控制DES中一直被忽视的H2O含量,起到稀释剂和络合剂的作用,避免DES凝固,实现Li的优先沉淀是至关重要的。对浸出和回收工艺的优化进行了全面研究,并在2 L浸出水平下进行了中等规模的试验,建立了浸出动力学模型。本文提出的策略为废旧锂离子电池直接、优先回收锂离子提供了良好的可能性,促进了电池回收市场经济高效、环保的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
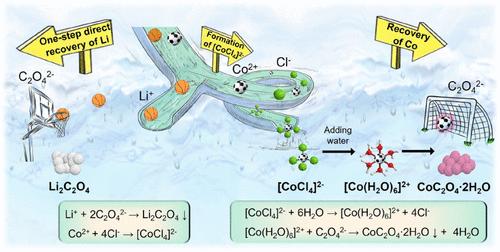
One-Step Direct Recovery of Lithium from Spent Lithium-Ion Batteries via Water-Based Deep Eutectic Solvent
The boom of the lithium-ion battery (LIB) industry is dialectically correlated with a scarcity of raw resources, particularly lithium (Li), so the recovery of critical metals from spent LIBs has become very essential for resource sustainability. Here, a new strategy for the one-step direct recovery of Li from spent LIB cathode active materials via water-based deep eutectic solvents (DESs) is put forward, together with the recycling and reuse of the DES. Without any reagent and pretreatment, Li2C2O4 can be directly and preferentially precipitated from the H2O-based choline chloride-oxalic acid (ChCl-OA) DES, and CoC2O4·2H2O is obtained easily from the residual Co-rich leachate by adding H2O. The leaching efficiencies are nearly 100% for Li and Co, and the recovery efficiencies reach 91.2 and 94.6%, respectively. The recovery mechanism of Li and Co is clarified based on the coordination-guided design between Li+ and C2O42–, which enables the precise separation of Li and Co. It is crucial to strictly control the H2O content in the DES, which is always being ignored, serving as a diluent and complexant, avoiding the solidification of the DES, and realizing the preferential precipitation of Li. The optimization of the leaching and recovery processes is comprehensively investigated as well as the middle-sized experiments in the 2 L leaching level, and a leaching kinetic model is established. The strategy proposed here offers a promising possibility for the direct and preferential recovery of Li from spent LIBs, promoting the development of high economic efficiency and environmental protection of the battery recycling market.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


