注射水凝胶辅助局部脂多糖递送改善免疫检查点阻断治疗。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
肿瘤相关巨噬细胞(tam)显著影响免疫检查点阻断(ICB)治疗的临床结果。旨在将tam从免疫抑制型M2表型重编程为促炎型M1表型的策略有望增强ICB疗效。脂多糖(LPS)是一种有效的toll样受体4 (TLR4)配体,可以将tam重编程为M1表型。然而,由于其明显的促炎特性,LPS的全身应用受到限制,这限制了其在癌症治疗中的安全剂量。为了解决这个问题,热敏水凝胶通过优化药物生物利用度和减少全身传播提供了一个可行的解决方案。在我们的研究中,将羧甲基壳聚糖(CS)加入Pluronic F127中,延长水凝胶的降解期,促进LPS在肿瘤部位的局部递送和积累。瘤周注射该水凝胶可增强抗pd -1抗体的抑瘤作用,显著提高4T1荷瘤小鼠的生存率。GelF127CS-LPS水凝胶还增加了肿瘤浸润树突状细胞活化标志物的表达,提高了M1/M2 TAM比例,增强了CD8+ T细胞向肿瘤的浸润,这是T细胞介导的抗肿瘤免疫的关键指标。值得注意的是,GelF127CS-LPS治疗未观察到明显的肝脏或血液学毒性,强调了其良好的安全性。这些发现证明了GelF127CS-LPS作为一种tams调节剂的潜力,以及一种有希望提高ICB治疗效果的组合策略。意义声明:LPS是一种有效的TLR4配体,可以将肿瘤相关巨噬细胞(tam)重编程为M1表型,从而有助于肿瘤抑制。但其抗肿瘤应用受到高剂量毒性与低剂量疗效不足的矛盾制约。为了解决这个问题,我们开发了一种包裹LPS的热敏水凝胶GelF127CS-LPS,它可以在肿瘤区域局部释放LPS。这种水凝胶在LPS的皮图水平上对tam进行重编程,以达到有利的M1/M2比例,促进T细胞介导的抗肿瘤免疫的激活,而没有明显的毒性。因此,当与免疫检查点阻断(ICB)联合使用时,水凝胶可以抑制肿瘤生长并提高总生存率。本研究提供了一种肿瘤靶向性治疗性LPS递送提高ICB疗效的有效方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
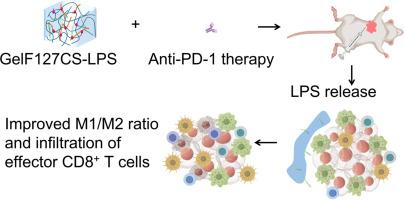
Injectable hydrogel-assisted local lipopolysaccharide delivery improves immune checkpoint blockade therapy
Tumor-associated macrophages (TAMs) significantly influence the clinical outcomes of immune checkpoint blockade (ICB) therapy. Strategies aimed at reprogramming TAMs from the immunosuppressive M2 phenotype to the pro-inflammatory M1 phenotype hold promise for enhancing ICB efficacy. Lipopolysaccharide (LPS), a potent Toll-like receptor 4 (TLR4) ligand, can reprogram TAMs toward an M1 phenotype. However, the systemic application of LPS is restricted due to its pronounced pro-inflammatory properties, which limit safe dosing in cancer treatment. To address this, thermosensitive hydrogels offer a viable solution by optimizing drug bioavailability and reducing systemic dissemination. In our study, carboxymethyl chitosan (CS) was incorporated into Pluronic F127 to extend the hydrogel's degradation period, facilitating the localized delivery and accumulation of LPS within tumor sites. Peritumoral injection of this hydrogel enhanced the tumor-inhibitory effects of anti-PD-1 antibodies, significantly improving the survival of 4T1 tumor-bearing mice. The GelF127CS-LPS hydrogel also increased the expression of the activation marker on tumor-infiltrating dendritic cells, promoted a higher M1/M2 TAM ratio, and enhanced CD8+ T cell infiltration into tumors—key indicators of T-cell-mediated anti-tumor immunity. Notably, no significant liver or hematological toxicity was observed with GelF127CS-LPS treatment, underscoring its favorable safety profile. These findings demonstrate the potential of GelF127CS-LPS as a TAMs-modulating agent and a promising combinatorial strategy to boost ICB therapy effectiveness.
Statement of significance
LPS, a potent TLR4 ligand, can reprogram tumor-associated macrophages (TAMs) toward an M1 phenotype, thereby contributing to tumor inhibition. However, its anti-tumor application is constrained by the contradiction between high-dose toxicity and insufficient efficacy at low doses. To address this issue, we developed a thermosensitive hydrogel encapsulating LPS, GelF127CS-LPS, which allows localized LPS release within the tumor area. This hydrogel reprograms TAMs at a picogram level of LPS to achieve a favorable M1/M2 ratio and promotes the activation of T cell-mediated antitumor immunity without observable toxicity. Consequently, when combined with immune checkpoint blockade (ICB), the hydrogel can inhibit tumor growth and improve overall survival. This study provides an effective method for tumor-targeted therapeutic LPS delivery to enhance the efficacy of ICB.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


