离子液体负载铜促进3,5-二取代-1,2,4-三唑的合成。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
三唑类化合物的合成在药物发现研究中起着重要作用。1,2,4-三唑被认为是几种生物活性杂环化合物中重要的支架,因为它们在制药和农用化学品领域得到了广泛的应用。因此,通过可持续的方法合成1,2,4-三唑的重要性增加了。在此,我们利用离子液体负载的铜(II)催化剂,在热加热和微波加热两种条件下,以苯腈衍生物和伯胺为原料合成1,2,4-三唑。我们的方法在相对较短的反应时间内提供了目标部分(7a-r)的优异收率。该合成方案的优点是通过可持续的方法,以简单的途径同时从容易获得的胺和腈中合成一对C-N键和一个N-N键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
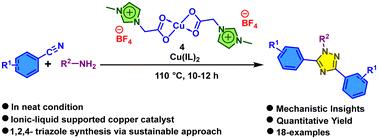
Ionic-liquid-supported copper-promoted synthesis of 3,5-disubstituted-1,2,4-triazoles†
The synthesis of triazoles plays an important role in drug discovery research. 1,2,4-triazoles are considered significant scaffolds among several bioactive heterocycles due to their extensive use in the pharmaceutical and agrochemical sectors. Consequently, the importance of the synthesis of 1,2,4-triazoles via a sustainable method has increased. Herein, we have utilized an ionic-liquid-supported copper(ii) catalyst for the synthesis of 1,2,4-triazoles from benzonitrile derivatives and primary amines under neat conditions both in thermal and microwave heating approaches. Our approach furnished excellent yields of the target moieties () in a comparatively short reaction time. This synthetic protocol provides the advantage of synthesizing a couple of C–N bonds and an N–N bond simultaneously from easily accessible amines and nitriles in a simple pathway via a sustainable approach.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


