Ag(I)-促进肽硫酰胺片段偶联。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
尽管在固相肽合成和肽连接方面取得了进展,但通过肽片段的偶联来组装多肽仍然存在挑战。本文描述了一种利用Ag(I)促进肽硫酰胺转化的肽片段偶联的新方法。该过程通过异亚胺系链中间体进行,中间体经过O-N酰基转移生成多肽。该方法既适用于固相耦合,也适用于固相耦合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
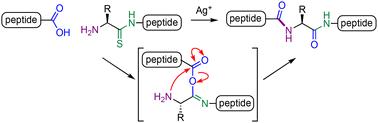
Ag(i)-promoted fragment coupling of peptide thioamides†
Despite advances in solid phase peptide synthesis and peptide ligation, challenges remain in the assembly of polypeptides through coupling of peptide fragments. Herein we describe a new method for peptide fragment coupling employing the Ag(i)-promoted transformation of peptide thioamides. This process proceeds via an isoimide-tethered intermediate, which undergoes an O–N acyl transfer to generate the polypeptide. This method is applicable to both solution- and solid-phase coupling.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


