铜通过α-羰基自由基催化芳基乙酸与甲基芳烃的C(sp3)-H/C(sp3)-H交叉偶联反应。
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
含吡啶基团的芳基乙酸等价物在铜基催化剂体系下与不同的甲基芳烃发生C(sp3)-H/C(sp3)-H交叉偶联。反应通过α-羰基自由基的形成而进行,从而获得α,β-二芳基丙酸。初步研究表明,该催化剂体系能够将芳基苄基酮转化为1,2,3-三芳基酮。本文章由计算机程序翻译,如有差异,请以英文原文为准。
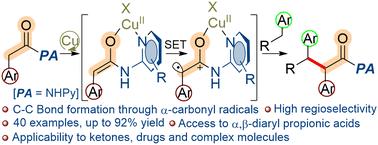
Copper catalyzed C(sp3)–H/C(sp3)–H cross-coupling of arylacetic acid equivalents with methylarenes via α-carbonyl radicals†
Arylacetic acid equivalents bearing a pyridine group undergo C(sp3)–H/C(sp3)–H cross coupling with diverse methylarenes in the presence of a copper based catalyst system. The reaction proceeds via the formation of α-carbonyl radicals giving access to α,β-diarylpropionic acids. Preliminary study suggests that the catalyst system is capable of transforming arylbenzyl ketones into 1,2,3-triaryl ketones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


