Pb2+ 6s2孤对活性的结构效应:偏心率
IF 20.3
1区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
6s2孤对的立体化学活性对Tl+、Pb2+和Bi3+等金属离子的配位化学具有深远的影响。Pb2+的配位化学很可能是在具有[Xe]4f145d106s2电子构型的金属离子中被研究得最广泛的,从而可以详细分析影响6s2孤对立体化学活性的因素。本文通过将Pb2+配合物的配位环境近似于一个长形球体,分析了多种Pb2+配合物的x射线结构。球体的偏心率(ε)提供了Pb2+孤对立体化学活性的直接测量。全向结构的特点是供体原子在金属离子周围呈球形分布,导致ε <;0.05. 孤对的立体化学活性可能导致中度偏定向结构(ε值在0.05 ~ 0.15之间)或强偏定向结构(ε >;0.15. 半定向结构受低配位数和带负电荷的供体原子的存在的青睐。本文不仅分析了许多具有代表性的配合物,并根据它们的偏心率对它们进行了分类,而且为配位化学家提供了一个简单的工具,使他们可以很容易地将配合物分类为全定向或半定向。本文章由计算机程序翻译,如有差异,请以英文原文为准。

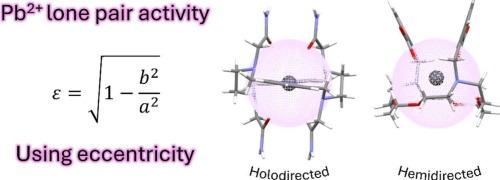
Structural effects of the Pb2+ 6s2 lone pair activity: Eccentricity
The stereochemical activity of the 6s2 lone pair has profound consequences on the coordination chemistry of metal ions like Tl+, Pb2+ and Bi3+. The coordination chemistry of Pb2+ is very likely the most extensively investigated among the metal ions with [Xe]4f145d106s2 electronic configuration, allowing for a detailed analysis of the factors that affect the stereochemical activity of the 6s2 lone pair. In this review, the X-ray structures of a wide variety of Pb2+ complexes were analyzed by approximating their coordination environments to a prolate spheroid. The eccentricity (ε) of the spheroid provides a straightforward measure of the stereochemical activity of the Pb2+ lone pair. Holodirected structures are characterized by a rather spherical distribution of the donor atoms around the metal ion, leading to values of ε < 0.05. The stereochemical activity of the lone pair may result in either moderately hemidirected structures, with ε values in the range 0.05–0.15, or strongly hemidirected structures, when ε > 0.15. Hemidirected structures are favored by low coordination numbers and the presence of negatively charged donor atoms. In this work not only have many representative complexes been analyzed to provide a classification of these systems according to their eccentricity, but also a simple tool is provided for coordination chemists, through which they can easily classify their complexes as holodirected or hemidirected.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Coordination Chemistry Reviews
化学-无机化学与核化学
CiteScore
34.30
自引率
5.30%
发文量
457
审稿时长
54 days
期刊介绍:
Coordination Chemistry Reviews offers rapid publication of review articles on current and significant topics in coordination chemistry, encompassing organometallic, supramolecular, theoretical, and bioinorganic chemistry. It also covers catalysis, materials chemistry, and metal-organic frameworks from a coordination chemistry perspective. Reviews summarize recent developments or discuss specific techniques, welcoming contributions from both established and emerging researchers.
The journal releases special issues on timely subjects, including those featuring contributions from specific regions or conferences. Occasional full-length book articles are also featured. Additionally, special volumes cover annual reviews of main group chemistry, transition metal group chemistry, and organometallic chemistry. These comprehensive reviews are vital resources for those engaged in coordination chemistry, further establishing Coordination Chemistry Reviews as a hub for insightful surveys in inorganic and physical inorganic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


