探讨Fe(Ⅵ)/PMS、Fe(Ⅵ)/PDS和Fe(Ⅵ)/SPC对污水溢流中氧氟沙星的降解作用:忽略氧化和原位混凝的协同作用
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
下水道溢流是城市水域新出现污染物的潜在来源,对生态系统和人类健康构成威胁。本文比较研究了高铁酸盐(Ⅵ)(Fe(Ⅵ))/过氧单硫酸酯(PMS)、Fe(Ⅵ)/过氧二硫酸酯(PDS)和Fe(Ⅵ)/过碳酸盐(SPC)对溢流中氧氟沙星(OFL)的降解性能和机理。这些系统实现了OFL的有效降解和常规污染物的去除。特别是Fe(Ⅵ)/PMS对OFL的降解效果更好,降解效率为98.8%。在Fe(Ⅵ)/PMS、Fe(Ⅵ)/PDS和Fe(Ⅵ)/SPC体系中,降解OFL的主要活性氧分别为单线态氧(1O2)、硫酸盐自由基(SO4·-)和羟基自由基(·OH)。高价铁在Fe(Ⅵ)/PMS和Fe(Ⅵ)/PDS体系中起重要作用。值得注意的是,氧化和原位混凝的协同作用对OFL的降解起着关键作用,这决定了Fe(Ⅵ)/PMS的优越性能。形成的具有Fe- o - p键的絮凝体作为高速公路,促进了电子从OFL向PMS的转移,从而实现了Fe(Ⅵ)/PMS体系中OFL的高效降解。此外,发现了OFL的相同降解途径,降解产物的毒性降低,特别是在Fe(Ⅵ)/PMS体系中。本研究为溢流治疗提供了新的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
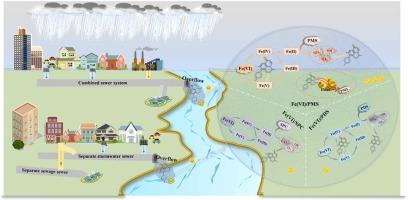
Exploring the degradation of ofloxacin in sewer overflows by Fe(Ⅵ)/PMS, Fe(Ⅵ)/PDS, and Fe(Ⅵ)/SPC: Overlooked synergistic effect of oxidation and in-situ coagulation
Sewer overflows are a potential source of emerging contaminants to urban waters, posing a threat to ecosystems and human health. Herein, the performance and mechanism of ferrate(Ⅵ) (Fe(Ⅵ))/peroxymonosulfate (PMS), Fe(Ⅵ)/peroxydisulfate (PDS), and Fe(Ⅵ)/percarbonate (SPC) for the degradation of ofloxacin (OFL) in overflows were comparatively investigated. These systems achieved efficient degradation of OFL and the removal of conventional pollutants. Particularly, Fe(Ⅵ)/PMS showed better degradation performance for OFL with a degradation efficiency of 98.8 %. The dominant reactive oxygen species for OFL degradation in the Fe(Ⅵ)/PMS, Fe(Ⅵ)/PDS, Fe(Ⅵ)/SPC systems were singlet oxygen (1O2), sulfate radical (SO4·-), and hydroxyl radical (·OH), respectively. High-valent iron species played an important role in the Fe(Ⅵ)/PMS and Fe(Ⅵ)/PDS systems. Notably, the synergistic effect of oxidation and in-situ coagulation played a key role in OFL degradation, which determined the superior performance of Fe(Ⅵ)/PMS. The formed flocs with Fe-O-P bond acted as a highway to promote the electron transfer from OFL to PMS, resulting in the efficient degradation of OFL in Fe(Ⅵ)/PMS system. Moreover, a same degradation pathway of OFL was found, and the toxicity of the degradation products was reduced, especially in the Fe(Ⅵ)/PMS system. This study provided a new strategy for overflows treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


