用淬灭策略在cu掺杂MnO2中构建氧空位以促进甲苯的催化氧化
IF 11.3
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
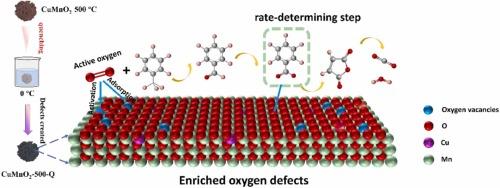
本文采用淬灭策略在Cu掺杂α-MnO2中形成氧空位。通过XRD细化、EPR和XPS直接跟踪了氧空位的演变。结合DFT计算和详细的表征,证据表明,氧空位不仅是气态氧和甲苯吸附和活化的直接场所,而且还通过削弱金属-氧键的强度加速晶格氧的消耗和补充周期。原位漂移研究表明,吸附氧和晶格氧都直接参与了甲苯的氧化分解,其中吸附氧在甲苯氧化生成苯甲酸酯的初始过程中起关键作用,而苯甲酸酯的开环过程依赖于晶格氧的活化。得益于氧空位对活性氧的重要贡献,淬火法制得的α-CuMnO2-500- q在240℃下能够完全催化甲苯的氧化,比原始α-CuMnO2-500还原了约80℃。此外,通过原位漂移光谱分析,提出了甲苯氧化机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Constructing oxygen vacancies in Cu-doped MnO2 by a quenching strategy for boosting the catalytic oxidation of toluene
Here, a quenching strategy was developed to create oxygen vacancies in Cu doped α-MnO2. The evolutions of oxygen vacancies were directly followed by means of XRD refinement, EPR and XPS. In combination with DFT calculations and detailed characterizations, evidence is captured that oxygen vacancies not only act as direct sites for the adsorption and activation of gaseous oxygen and toluene, but also accelerate the consumption and replenishment cycle of lattice oxygen species by weakening the strength of metal-oxygen bonds. In situ DRIFTS study reveals that both adsorbed oxygen and lattice oxygen species directly participate in the oxidative decomposition of toluene, where adsorbed oxygen species play pivotal roles in the initial oxidation of toluene to benzoate, whereas the process of ring opening of benzoate relies on the activation of lattice oxygen. Benefiting from crucial contribution of oxygen vacancies in activating oxygen species, α-CuMnO2-500-Q obtained by the quenching method is capable of fully catalyzing the oxidation of toluene at 240 ℃, representing a reduction of about 80 ℃ compared to pristine α-CuMnO2-500. Furthermore, the toluene oxidation mechanism was proposed as well via in situ DRIFT spectra.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


