双特异性适配体-药物偶联物选择性消除恶性血液细胞治疗急性髓系白血病
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
表面抗原定向免疫治疗是急性髓性白血病(AML)的一种根治性治疗方式,其特点是表面抗原的丰富和稳定表达。然而,目前表面抗原导向的免疫疗法在治疗AML患者方面显示出较差的结果和不理想的死亡率,主要是由于使用单靶点治疗来解决表面抗原的异质表达而产生的获得性耐药。因此,为了提高抗原特异性治疗AML的疗效,我们设计了一种双特异性适配体-药物偶联物。特别是,将cell lysate-SELEX用于适配体与HEL细胞结合的cell- selex产生了AptCD117,它特异性地结合CD117(造血干细胞和原代AML细胞上高度表达的标记物),并且在靶向人类AML细胞方面具有出色的性能。与cd71结合的适配体LXD-11b(白血病细胞上另一种广泛表达的表面抗原)结合,设计双特异性适配体与单甲基auristatin F (MMAF)偶联,制备适配体-药物偶联物。结果表明,双特异性适配体- mmaf偶联物在体外可有效杀伤不同CD117和CD71表达水平的靶AML细胞系。重要的是,将AML骨髓标本暴露于双特异性适配体- mmaf偶联物中,可在体外选择性消除原代AML细胞,而对同一标本中的健康淋巴细胞没有影响。因此,这些结果为产生针对人类AML细胞的双特异性适配体-药物偶联物提供了概念证明,这有望推进治疗策略并改善AML患者的预后。本文章由计算机程序翻译,如有差异,请以英文原文为准。
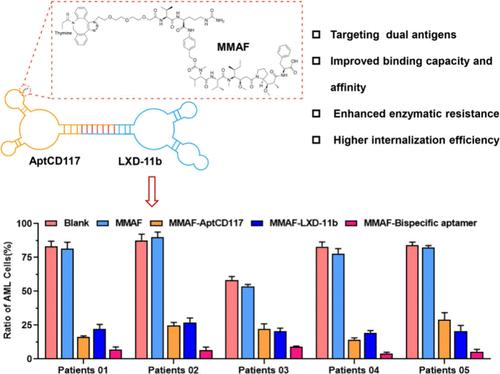
Bispecific Aptamer-Drug Conjugates Selectively Eliminate Malignant Hematologic Cells for Treating Acute Myeloid Leukemia
Surface antigen-directed immunotherapy is a curative treatment modality for acute myeloid leukemia (AML) that is characterized by the abundance and stability expression of surface antigens. However, current surface antigen-directed immunotherapies have shown poor outcomes and undesirable mortality rates in treating AML patients, primarily due to acquired resistance that arises from using single-target therapies to address the heterogeneous expression of surface antigens. Hence, in order to improve the efficacy of antigen-specific therapies for treating AML, we designed a bispecific aptamer-drug conjugate. In particular, cell-SELEX incorporating cell lysate-SELEX for aptamers with HEL cells yielded AptCD117, which specifically binds to CD117 (a highly expressed marker on both hematopoietic stem cells and primary AML cells) and has excellent performance in targeting human AML cells. Combined with CD71-binding aptamer LXD-11b (another broadly expressed surface antigen on leukemia cells), bispecific aptamers were designed to couple with monomethyl auristatin F (MMAF) for fabricating aptamer-drug conjugates. Results demonstrated that bispecific aptamer-MMAF conjugates efficiently kill different CD117 and CD71 expression levels of target AML cell lines in vitro. Importantly, the exposure of AML marrow specimens to bispecific aptamer-MMAF conjugates resulted in the selective elimination of primary AML cells in vitro and had no effect on healthy lymphocytes within the same specimens. Thus, these results provide a proof of concept for the generation of bispecific aptamer-drug conjugates directed against human AML cells, which hold the promise of advancing treatment strategies and improving AML patient outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


