壳聚糖修饰的磁性生物炭对全氟辛酸的吸附:基于响应面法的建模、性能和机理
IF 7.6
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
全氟辛酸(PFOA)的去除由于其环境稳定性和潜在毒性而受到广泛关注。本研究旨在合成壳聚糖修饰的磁性生物炭(CS_MBC),以高效去除水溶液中的PFOA。通过对不同CS加载比例(0.25:1、0.5:1和1:1)的研究,确定了最佳吸附剂,初步实验表明CS1_MBC具有较好的吸附性能。为了探索不同实验条件(pH、吸附剂剂量、时间和初始PFOA浓度)对PFOA去除效果的影响,采用响应面法的中心复合设计进行优化。通过方差统计分析来评估模型的充分性,实验结果与模型有较强的相关性。预测达到最大PFOA去除率(~ 94%)的最佳条件为pH 4、120 mg/L剂量、60 min时间和20 mg/L PFOA浓度。动力学和等温线研究表明,拟二阶(R2=0.9996)和Redlich-Peterson (R2=0.999)模型较好地描述了PFOA的吸附,Langmuir最大吸附量为~ 517 mg/g。热力学研究证实了PFOA吸附的自发、吸热和物理性质,静电和疏水相互作用以及氢键控制了这一过程。通过固定床柱实验对CS1_MBC的实际应用效果进行了评价,实验结果表明,CS1_MBC的最大吸附量为39.63 mg/g。突破数据与Thomas模型和Yoon-Nelson模型拟合良好,相关系数高(R2=0.99)。因此,本研究强调了CS_MBC作为一种有效的吸附剂在水环境中减轻PFOA污染的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。

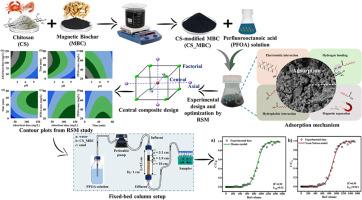
Adsorption of perfluorooctanoic acid from aqueous matrices onto chitosan-modified magnetic biochar: Response surface methodology-based modeling, performance, and mechanism
Perfluorooctanoic acid (PFOA) removal has gained significant attention due to its environmental stability and potential toxicity. This study aims to synthesize a chitosan-modified magnetic biochar (CS_MBC) for efficient PFOA removal from aqueous solutions. Various CS loading ratios (0.25:1, 0.5:1, and 1:1) were explored to determine the optimal adsorbent, with preliminary experiments exhibiting superior performance of CS1_MBC. To explore the impact of various experimental conditions (pH, adsorbent dose, time, and initial PFOA concentrations) on PFOA removal and optimize these parameters, central composite design of response surface methodology was applied. Statistical analysis of variance was conducted to assess the model's adequacy, which demonstrated a strong correlation between experimental results and the model. The predicted optimal conditions for achieving maximum PFOA removal (∼94%) were pH 4, 120 mg/L dose, 60 min time, and 20 mg/L PFOA concentration. The kinetics and isotherm studies revealed that the pseudo-second-order (R2 = 0.9996) and Redlich-Peterson (R2 = 0.999) models better described PFOA adsorption, with Langmuir maximum adsorption capacity of ∼517 mg/g. Thermodynamic study confirmed the spontaneous, endothermic, and physisorptive nature of PFOA adsorption, with electrostatic and hydrophobic interactions and hydrogen bonding governing the process. Further, the fixed-bed column experiment was conducted to evaluate the effectiveness of CS1_MBC for practical applications, which demonstrated the maximum experimental adsorption capacity of 39.63 mg/g. The breakthrough data showed an excellent fit with both the Thomas and Yoon-Nelson models, with a high correlation coefficient (R2 = 0.99). Therefore, this research underscores the potential of CS_MBC as an efficient adsorbent for mitigating PFOA contamination in aqueous environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


