对称双腙配体双核氧化/二氧化钒(IV/V)配合物:合成、反应活性及其在合成具有生物活性的2-苯基喹唑啉-4-(3H)-的催化应用
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
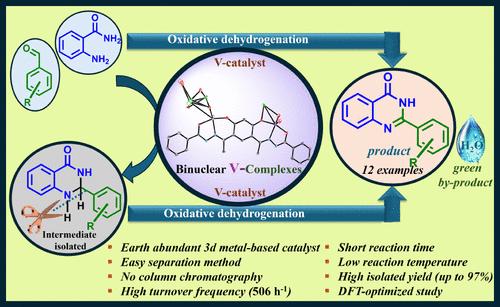
由4,6-二乙酰间苯二酚(H2dar)和不同的肼[苯甲酰肼(Hbhz)、异烟碱酰肼(Hinh)、烟碱酰肼(Hnah)和2-呋喃酰肼(Hfah)]合成对称的双(腙)基配体H4dar(bhz)2 (I)、H4dar(nah)2 (III)和H4dar(inh)2 (IV)),用于制备双核顺式-[VVO2]+配合物的钾盐,{K(H2O)2}2[(VVO2)2dar(bhz)2](1)、{K(H2O)2}2[(VVO2)2dar(fah)2](2)、{K(H2O)2}2[(VVO2)2dar(fah)2](3)、和{K(H2O)2}2[(VVO2)2dar(inh)2](4),以及双核[VIVO]2+配合物[{VIVO(MeOH)}2dar(bhz)2] (5), [{VIVO(MeOH)}2dar(fah)2] (6), [{VIVO(MeOH)}2dar(nah)2](7)和[{VIVO(MeOH)}2dar(inh)2](8)。在温水/DMSO(4:1)存在下,3变为{K(H2O)2}[(VVO2)2Hdar(nah)2]·DMSO (3a·DMSO)。1和3a的单晶XRD研究证实了其双核结构以及每个钒中心扭曲的方形锥体几何形状,其中其{ONO(2−)}配体通过每个单元的酚- o,偶氮- n和烯醇- o原子进行配位。在EtOH中生长6晶体时,部分氧化生成[{VVO(OEt)}2dar(fah)2](9)和粉末6。配合物9具有扭曲的八面体结构。这些配合物作为催化剂用于合成具有重要生物意义的2-苯基喹唑啉-4-(3H)-具有不同芳基醛的化合物,在70% TBHP/30% H2O2作为绿色氧化剂存在的条件下,均在较短的反应时间和较低的温度下表现出优异的催化性能(产率高达97%)。一般来说,这些配合物比它们的单核类似物性能更好。光谱学、DFT研究和分离的中间体有助于提出催化反应的合适反应机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Symmetrical Bis-Hydrazone Ligand-Based Binuclear Oxido/Dioxido-Vanadium(IV/V) Complexes: Synthesis, Reactivity, and Catalytic Applications for the Synthesis of Biologically Potent 2-Phenylquinazolin-4-(3H)-ones
Symmetrical bis(hydrazone)-based ligands, H4dar(bhz)2 (I), H4dar(fah)2 (II), H4dar(nah)2 (III), and H4dar(inh)2 (IV) obtained from 4,6-diacetylresorcinol (H2dar) and different hydrazides [benzoylhydrazide (Hbhz), isonicotinoylhydrazide (Hinh), nicotinoylhydrazide (Hnah), and 2-furoylhydrazide (Hfah)], were used to prepare potassium salts of binuclear cis-[VVO2]+ complexes, {K(H2O)2}2[(VVO2)2dar(bhz)2] (1), {K(H2O)2}2[(VVO2)2dar(fah)2] (2), {K(H2O)2}2[(VVO2)2dar(nah)2] (3), and {K(H2O)2}2[(VVO2)2dar(inh)2] (4), and binuclear [VIVO]2+ complexes, [{VIVO(MeOH)}2dar(bhz)2] (5), [{VIVO(MeOH)}2dar(fah)2] (6), [{VIVO(MeOH)}2dar(nah)2] (7), and [{VIVO(MeOH)}2dar(inh)2] (8). In the presence of warm MeOH/DMSO (4:1), 3 changed to {K(H2O)2}[(VVO2)2Hdar(nah)2]·DMSO (3a·DMSO). Single crystal XRD studies of 1 and 3a confirm a binuclear structure along with a distorted square pyramidal geometry of each vanadium center where bis{ONO(2−)} ligands coordinate through phenolate-O, azomethine-N, and enolate-O atoms of each unit. While growing crystals of 6 in EtOH, part of it oxidizes and gives [{VVO(OEt)}2dar(fah)2] (9) along with powdery 6. Complex 9 has a distorted octahedral structure. These complexes were used as catalysts for the synthesis of biologically important 2-phenylquinazolin-4-(3H)-ones having different aryl aldehydes, and they all show excellent catalytic performance (up to 97% yield) in less reaction time and low temperature, in the presence of 70% aqueous TBHP/30% aqueous H2O2 as a greener oxidant. Generally, these complexes perform better than their mononuclear analogues. Spectroscopy, DFT studies, and isolated intermediates have helped in proposing a suitable reaction mechanism for the catalytic reaction.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


