通过合理设计的醛酸酶对(R)-和(S)-杂芳基醛醇进行对映异构合成
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
醛缩酶,特别是2-脱氧核糖-5-磷酸醛缩酶(DERA)已被广泛应用于获取各种活性药物成分(api)的关键手性前体。这是通过蛋白质工程将它们的底物范围扩展到非天然受体和供体而实现的。本研究试图通过合理的蛋白工程方法,拓宽Geobacillus sp. (DERAGeo)中DERA对杂芳醛的受体底物范围。我们成功地对DERAGeo进行了迭代饱和诱变,产生了两个对映互补变体,即(R)选择性DERAGeo- s185g和(S)选择性DERAGeo- t12i /S185A,具有增强的催化效率和对映选择性。值得注意的是,DERAGeo的天然对映体选择性被单个突变(S185G)逆转。通过在半制备规模上进行醛醇反应,证明了突变体的合成适用性,从中分离出杂芳醛醇的(R)-和(S)-对映体,收率高(高达99%),对映纯度高(高达99:1 e.r)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
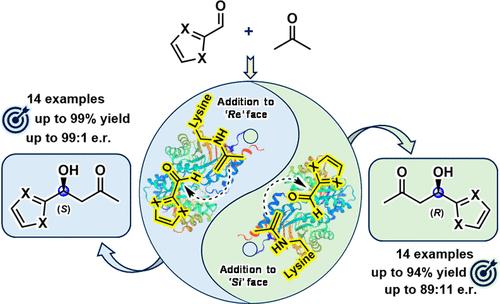
Enantiodivergent Synthesis of Both (R)- and (S)-Heteroaryl Aldols by Rationally Engineered Aldolases
Aldolases, especially 2-deoxyribose-5-phosphate aldolase (DERA) enzymes, have been widely employed to access key chiral precursors for various active pharmaceutical ingredients (APIs). This has been enabled by expanding their substrate scope toward non-natural acceptors and donors via protein engineering. In this study, we endeavored to broaden the acceptor substrate scope of DERA from Geobacillus sp. (DERAGeo) toward the heteroaryl aldehydes through a rational protein engineering approach. We successfully performed iterative saturation mutagenesis of DERAGeo, resulting in two enantiocomplementary variants, viz., (R)-selective DERAGeo-S185G and (S)-selective DERAGeo-T12I/S185A, with enhanced catalytic efficiencies and enantioselectivities. Remarkably, the natural enantioselectivity of DERAGeo was reversed by a single mutation (S185G). The synthetic applicability of the mutants was demonstrated by conducting aldol reactions on a semipreparative scale, from which both (R)- and (S)-enantiomers of heteroaryl aldols were isolated with high yields (up to 99%) and excellent enantiopurities (up to 99:1 e.r.).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


