稳定锌水电池的弱氢键界面环境
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
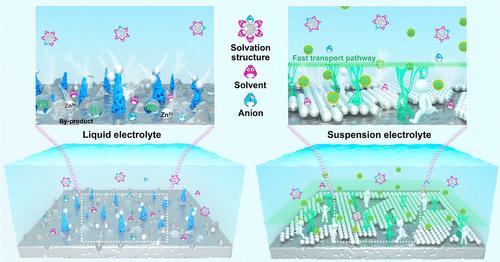
由于高水活度和电化学动力学与传质之间的不利竞争,析氢反应和Zn枝晶生长是制约水性锌基电池商业化应用的主要因素。本文通过在电解质中加入TiO2纳米粒子,建立了与悬浮电解质的弱氢键界面。由于TiO2中Ti-O键的强极性,在TiO2[110]活性表面与水环境之间形成了丰富的羟基官能团,通过破坏水分子间的初始氢键网络产生弱氢键界面,从而加速Zn2+的传质,降低水活性。因此,锌||锌对称电池表现出可逆的镀锌/剥离行为,在700次循环中库仑效率高达99.7%。此外,基于tio2的悬浮策略也适用于其他锌盐体系,并表现出快速的镀/剥离行为。悬浮电解质可实现长期充满电池,包括Zn||PANI混合电容器和Zn||ZnVO充满电池。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Weak H-Bond Interface Environment for Stable Aqueous Zinc Batteries
Hydrogen evolution reaction and Zn dendrite growth, originating from high water activity and the adverse competition between the electrochemical kinetics and mass transfer, are the main constraints for the commercial applications of the aqueous zinc-based batteries. Herein, a weak H-bond interface with a suspension electrolyte is developed by adding TiO2 nanoparticles into the electrolytes. Owing to the strong polarity of Ti–O bonds in TiO2, abundant hydroxyl functional groups are formed between the TiO2[110] active surface and aqueous environment, which can produce a weak H-bond interface by disrupting the initial H-bond networks between the water molecules, thereby accelerating the mass transfer of Zn2+ and reducing the water activity. In consequence, the Zn||Zn symmetrical cells display reversible Zn plating/stripping behaviors with a high Coulombic efficiency of 99.7% over 700 cycles. Moreover, the TiO2-based suspension strategy is also applicable to other zinc salt systems and exhibits fast plating/stripping behaviors. The suspension electrolyte enables long-term full cells, including Zn||PANI hybrid capacitors and Zn||ZnVO full batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


