具有增强超氧化物歧化酶样活性的普鲁士蓝纳米酶治疗心肌缺血再灌注损伤
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
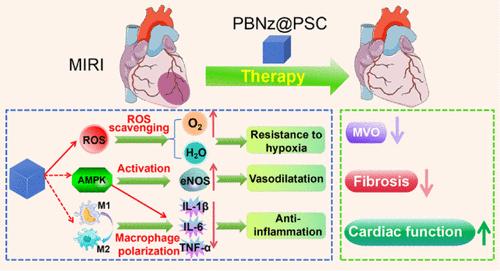
当临床上心肌梗死(MI)后的血流恢复时,会破坏缺血心肌区域的生理和代谢平衡,导致继发性损伤,称为心肌缺血再灌注损伤(MIRI)。活性氧(ROS)的产生和炎症反应是MIRI的主要原因。目前侧重于清除ros和抗炎作用的策略对MIRI的缓解有限。普鲁士蓝纳米酶(PBNz)具有多种酶样活性,包括过氧化氢酶(CAT)、过氧化物酶(POD)和超氧化物歧化酶(SOD),有助于清除活性氧和抗炎症。本文开发了一种包被聚葡萄糖-山梨醇羧甲基醚(PBNz@PSC)的PBNz配方,以提高其治疗MIRI的疗效、生物相容性和安全性。PBNz@PSC不仅由于其多糖属性而表现出增强的sod样活性,而且可以通过增强渗透性和潴留(EPR)作用被动靶向受损心肌。体外和体内研究都证实了其良好的生物相容性、安全性、ros清除能力,以及驱动巨噬细胞从M1向M2极化的能力,从而降低IL-1β、IL-6和TNF-α的水平,以对抗炎症。因此,PBNz@PSC可以逆转缺血再灌注引起的心肌损伤,减少冠状动脉微血管阻塞(MVO),改善心肌重构和心功能。此外,PBNz@PSC对MIRI的治疗效果比临床药物磺胺丹参酮IIA钠更明显。值得注意的是,我们的研究结果揭示了PBNz@PSC治疗介导AMPK激活的MIRI的可能机制。总之,本研究提出了一种解决MIRI的开创性策略,有望改善缺血再灌注结果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Prussian Blue Nanozyme Featuring Enhanced Superoxide Dismutase-like Activity for Myocardial Ischemia Reperfusion Injury Treatment
The blood flow, when restored clinically following a myocardial infarction (MI), disrupts the physiological and metabolic equilibrium of the ischemic myocardial area, resulting in secondary damage termed myocardial ischemia-reperfusion injury (MIRI). Reactive oxygen species (ROS) generation and inflammatory reactions stand as primary culprits behind MIRI. Current strategies focusing on ROS-scavenging and anti-inflammatory actions have limited remission of MIRI. Prussian blue nanozyme (PBNz) exhibits multiple enzyme-like activities including catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD), which are beneficial for ROS clearance and fighting inflammation. Herein, a formulation of PBNz coated with polydextrose-sorbitol carboxymethyl ether (PBNz@PSC) was developed to enhance its efficacy, biocompatibility, and safety for the treatment of MIRI. PBNz@PSC not only showed enhanced SOD-like activity due to its polysaccharide attributes but also could passively target the damaged myocardium through the enhanced permeability and retention (EPR) effect. Both in vitro and in vivo studies have validated their excellent biocompatibility, safety, ROS-scavenging ability, and capacity to drive macrophage polarization from M1 toward M2, thereby diminishing the levels of IL-1β, IL-6, and TNF-α to combat inflammation. Consequently, PBNz@PSC can reverse ischemia reperfusion-induced myocardial injury, reduce coronary microvascular obstruction (MVO), and improve myocardial remodeling and cardiac function. Moreover, PBNz@PSC showed more pronounced therapeutic effects for MIRI than a clinical drug, sulfotanshinone IIA sodium. Notably, our findings revealed the possible mechanism of PBNz@PSC in treating MIRI, which mediated AMPK activation. In conclusion, this study presents a pioneering strategy for addressing MIRI, promising improved ischemia-reperfusion outcomes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


